No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting
- PMID: 24797938
- PMCID: PMC4010496
- DOI: 10.1371/journal.pone.0096567
No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting
Erratum in
- PLoS One. 2014;9(8):e105663
Abstract
Objective: Remote ischemic preconditioning (RIPC) by repeated brief limb ischemia/reperfusion reduces myocardial injury in patients undergoing coronary artery bypass grafting (CABG). Activation of signal transducer and activator of transcription 5 (STAT5) in left ventricular (LV) myocardium at early reperfusion is associated with such protection. Autophagy, i.e., removal of dysfunctional cellular components through lysosomes, has been proposed as one mechanism of cardioprotection. Therefore, we analyzed whether or not the protection by RIPC is associated with activated autophagy.
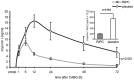
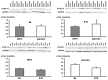
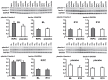
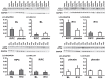
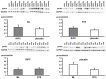
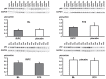
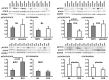
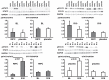
Methods: CABG patients were randomized to undergo RIPC (3×5 min blood pressure cuff inflation/5 min deflation) or placebo (cuff deflated) before skin incision (n = 10/10). Transmural myocardial biopsies were taken from the LV before cardioplegia (baseline) and at early (5-10 min) reperfusion. RIPC-induced protection was reflected by decreased serum troponin I concentration area under the curve (194±17 versus 709±129 ng/ml × 72 h, p = 0.002). Western blotting for beclin-1-phosphorylation and protein expression of autophagy-related gene 5-12 (ATG5-12) complex, light chain 3 (LC3), parkin, and p62 was performed. STAT3-, STAT5- and extracellular signal-regulated protein kinase 1/2 (ERK1/2)-phosphorylation was used as positive control to confirm signal activation by ischemia/reperfusion.
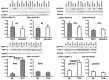
Results: Signals of all analyzed autophagy proteins did not differ between baseline and early reperfusion and not between RIPC and placebo. STAT5-phosphorylation was greater at early reperfusion only with RIPC (2.2-fold, p = 0.02). STAT3- and ERK1/2-phosphorylation were greater at early reperfusion with placebo and RIPC (≥2.7-fold versus baseline, p≤0.05).
Conclusion: Protection through RIPC in patients undergoing CABG surgery does not appear to be associated with enhanced autophagy in LV myocardium at early reperfusion.
Conflict of interest statement
Figures
References
-
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, et al. (2007) Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet 370: 575–579. - PubMed
-
- Venugopal V, Hausenloy DJ, Ludman A, Di Salvo CM, Kolvekar S, et al. (2009) Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold blood cardioplegia: a randomised controlled trial. Heart 95: 1567–1571. - PubMed
-
- Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, et al. (2010) Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 105: 657–664. - PubMed
-
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, et al. (2013) Cardioprotection and prognosis by remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 382: 597–604. - PubMed
-
- Heusch G (2013) Cardioprotection – chances and challenges of its translation to the clinic. Lancet 381: 166–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

