Reciprocal regulation of reactive oxygen species and phospho-CREB regulates voltage gated calcium channel expression during Mycobacterium tuberculosis infection
- PMID: 24797940
- PMCID: PMC4010530
- DOI: 10.1371/journal.pone.0096427
Reciprocal regulation of reactive oxygen species and phospho-CREB regulates voltage gated calcium channel expression during Mycobacterium tuberculosis infection
Abstract
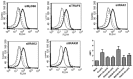
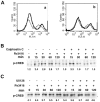
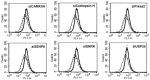
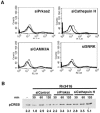
Our previous work has demonstrated the roles played by L-type Voltage Gated Calcium Channels (VGCC) in regulating Mycobacterium tuberculosis (M. tb) survival and pathogenesis. Here we decipher mechanisms and pathways engaged by the pathogen to regulate VGCC expression in macrophages. We show that M. tb and its antigen Rv3416 use phospho-CREB (pCREB), Reactive Oxygen Species (ROS), Protein Kinase C (PKC) and Mitogen Activated Protein Kinase (MAPK) to modulate VGCC expression in macrophages. siRNA mediated knockdown of MyD88, IRAK1, IRAK2 or TRAF6 significantly inhibited antigen mediated VGCC expression. Inhibiting Protein Kinase C (PKC) or MEK-ERK1/2 further increased VGCC expression. Interestingly, inhibiting intracellular calcium release upregulated antigen mediated VGCC expression, while inhibiting extracellular calcium influx had no significant effect. siRNA mediated knockdown of transcription factors c-Jun, SOX5 and CREB significantly inhibited Rv3416 mediated VGCC expression. A dynamic reciprocal cross-regulation between ROS and pCREB was observed that in turn governed VGCC expression with ROS playing a limiting role in the process. Further dissection of the mechanisms such as the interplay between ROS and pCREB would improve our understanding of the regulation of VGCC expression during M. tb infection.
Conflict of interest statement
Figures




References
-
- World Health Organization (2012) Global tuberculosis control 2012. Available: www.who.org.
-
- Koul A, Herget T, Klebl B, Ullrich A (2004) Interplay between Mycobacteria and host signaling pathways. . Nat. Rev. Microbiol. 2: 189–202. - PubMed
-
- Cantrell D (1996) T cell antigen receptor signal pathways. . Ann. Rev Immunol.14: 259–274. - PubMed
-
- Berridge MJ (1993) Cell signaling. A tale of two messengers. Nature 365: 388–389. - PubMed
-
- Lewis RS (2007) The molecular choreography of a store-operated calcium channel. Nature 446: 284–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

