Medication monitoring in a nurse-led respiratory outpatient clinic: pragmatic randomised trial of the West Wales Adverse Drug Reaction Profile
- PMID: 24798210
- PMCID: PMC4010491
- DOI: 10.1371/journal.pone.0096682
Medication monitoring in a nurse-led respiratory outpatient clinic: pragmatic randomised trial of the West Wales Adverse Drug Reaction Profile
Abstract
Objective: To assess the clinical effect of medication monitoring using the West Wales Adverse Drug Reaction (ADR) Profile for Respiratory Medicine.
Design: Single-site parallel-arm pragmatic trial using stratified randomisation.
Setting: Nurse-led respiratory outpatient clinic in general hospital in South Wales.
Participants: 54 patients with chronic respiratory disease receiving bronchodilators, corticosteroids or leukotriene receptor antagonists.
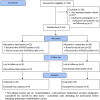
Intervention: Following initial observation of usual nursing care, we allocated participants at random to receive at follow up: either the West Wales ADR Profile for Respiratory Medicine in addition to usual care ('intervention arm' with 26 participants); or usual care alone ('control arm' with 28 participants).
Main outcome measures: Problems reported and actions taken.
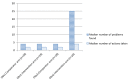
Results: We followed up all randomised participants, and analysed data in accordance with treatment allocated. The increase in numbers of problems per participant identified at follow up was significantly higher in the intervention arm, where the median increase was 20.5 [inter-quartile range (IQR) 13-26], while that in the control arm was -1 [-3 to +2] [Mann-Whitney U test: z = 6.28, p<0.001]. The increase in numbers of actions per participant taken at follow up was also significantly higher in the intervention arm, where the median increase was 2.5 [1]-[4] while that in the control arm was 0 [-1.75 to +1] [Mann-Whitney U test: z = 4.40, p<0.001].
Conclusion: When added to usual nursing care, the West Wales ADR Profile identified more problems and prompted more nursing actions. Our ADR Profile warrants further investigation as a strategy to optimise medication management.
Trial registration: Controlled-trials.com ISRCTN10386209.
Conflict of interest statement
Figures
Similar articles
-
Development and clinical gains of nurse-led medication monitoring profiles.J Nurs Manag. 2014 Apr;22(3):331-49. doi: 10.1111/jonm.12067. Epub 2013 May 23. J Nurs Manag. 2014. PMID: 23701013
-
Nurse-Led Medicines' Monitoring for Patients with Dementia in Care Homes: A Pragmatic Cohort Stepped Wedge Cluster Randomised Trial.PLoS One. 2015 Oct 13;10(10):e0140203. doi: 10.1371/journal.pone.0140203. eCollection 2015. PLoS One. 2015. PMID: 26461064 Free PMC article. Clinical Trial.
-
Medication monitoring for people with dementia in care homes: the feasibility and clinical impact of nurse-led monitoring.ScientificWorldJournal. 2014 Feb 23;2014:843621. doi: 10.1155/2014/843621. eCollection 2014. ScientificWorldJournal. 2014. PMID: 24707218 Free PMC article.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.Health Technol Assess. 2005 Feb;9(3):iii-iv, 1-126. doi: 10.3310/hta9030. Health Technol Assess. 2005. PMID: 15694064 Review.
-
Monitoring adverse drug reactions: scales, profiles, and checklists.Int Nurs Rev. 2004 Dec;51(4):208-21. doi: 10.1111/j.1466-7657.2004.00251.x. Int Nurs Rev. 2004. PMID: 15530161 Review.
Cited by
-
Reducing Adverse Drug Reactions for Older People in the Community: Evaluating the Validity and Reliability of the ADRe Profile.J Nurs Manag. 2025 May 14;2025:9921349. doi: 10.1155/jonm/9921349. eCollection 2025. J Nurs Manag. 2025. PMID: 40401039 Free PMC article. Clinical Trial.
-
Variation of adverse drug events in different settings in Africa: a systematic review.Eur J Med Res. 2024 Jun 16;29(1):333. doi: 10.1186/s40001-024-01934-0. Eur J Med Res. 2024. PMID: 38880895 Free PMC article.
-
Nurse-led medicines' monitoring in care homes study protocol: a process evaluation of the impact and sustainability of the adverse drug reaction (ADRe) profile for mental health medicines.BMJ Open. 2018 Sep 28;8(9):e023377. doi: 10.1136/bmjopen-2018-023377. BMJ Open. 2018. PMID: 30269073 Free PMC article.
-
A qualitative exploration of patient safety in a hospital setting in Spain: Policy and practice recommendations on patients' and companions' participation.Health Expect. 2023 Aug;26(4):1536-1550. doi: 10.1111/hex.13758. Epub 2023 Apr 14. Health Expect. 2023. PMID: 36971145 Free PMC article.
-
Adverse Drug Reactions, Power, Harm Reduction, Regulation and the ADRe Profiles.Pharmacy (Basel). 2018 Sep 18;6(3):102. doi: 10.3390/pharmacy6030102. Pharmacy (Basel). 2018. PMID: 30231573 Free PMC article.
References
-
- Baines RJ, Langelaan M, de Bruijne MC, Asscheman H, Spreeuwenberg P, et al. (2013) Changes in adverse event rates in hospitals over time: a longitudinal retrospective record review. Quality and Safety in Health Care 22: 290–298. - PubMed
-
- Aronson JK (2013) Adverse drug reactions: history, terminology, classification, causality, frequency, preventability In: Talbot J, Aronson JK, editors. Stephens’ Detection and Evaluation of Adverse Drug Reactions: Principles and Practice. 6th edtn. Chichester, UK: Wiley-Blackwell 1–119.
-
- International Conference of Harmonization (1996) ICH Harmonised Tripartite Guideline for Good Clinical Practice. Buckinghamshire, UK: Institute of Clinical Research. Available: http://www.ich.org/cache/compo/475-272-1.html#E6 (accessed 09 September 2010)
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials