Surotomycin demonstrates low in vitro frequency of resistance and rapid bactericidal activity in Clostridium difficile, Enterococcus faecalis, and Enterococcus faecium
- PMID: 24798273
- PMCID: PMC4068600
- DOI: 10.1128/AAC.00124-14
Surotomycin demonstrates low in vitro frequency of resistance and rapid bactericidal activity in Clostridium difficile, Enterococcus faecalis, and Enterococcus faecium
Abstract
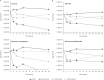
Surotomycin (CB-183,315) is an orally administered, minimally absorbed, selective bactericidal cyclic lipopeptide in phase 3 development for the treatment of Clostridium difficile-associated diarrhea. The aim of this study was to evaluate the emergence of resistance in C. difficile (ATCC 700057 and three recent clinical isolates from the restriction endonuclease analysis groups BI, BK, and K), vancomycin-susceptible (VS) Enterococcus faecalis (ATCC 49452), vancomycin-resistant (VR) E. faecalis (ATCC 700802), VS Enterococcus faecium (ATCC 6569), and VR E. faecium (ATCC 51559) under anaerobic conditions. The rate of spontaneous resistance was below the limit of detection (<10(-8) to <10(-9)) for surotomycin at 16 and 32× the MIC for all isolates tested. Under selective pressure by serial passage, C. difficile grew in a maximum of 4 μg/ml surotomycin (final MICs of 2 to 8 μg/ml [4- to 16-fold higher than those of the naive control]) at day 15, with the exception of the C. difficile BK strain, which grew in 16 to 32 μg/ml (final MICs of 8 to 32 μg/ml [16- to 64-fold higher than those of the naive control]). Enterococci remained relatively unchanged over 15 days, growing in a maximum of 8 μg/ml surotomycin (final MICs of 2 to 16 μg/ml [8- to 64-fold higher than those of the naive control]). Of the isolates tested, no cross-resistance to vancomycin, rifampin, ampicillin, metronidazole, or moxifloxacin was observed. Surotomycin at 20× MIC demonstrated equally rapid bactericidal activity (≥ 3-log-unit reduction in CFU/ml in ≤ 8 h) against naive and reduced-susceptibility isolates of C. difficile, VS Enterococcus (VSE), and VR Enterococcus (VRE), except for C. difficile BK (2.6-log-unit reductions for both). These results suggest that emergence of resistance to surotomycin against C. difficile, E. faecalis, and E. faecium is likely to be rare.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

