Live cell imaging reveals differential modifications to cytoplasmic dynein properties by phospho- and dephosphomimic mutations of the intermediate chain 2C S84
- PMID: 24798412
- PMCID: PMC4107179
- DOI: 10.1002/jnr.23388
Live cell imaging reveals differential modifications to cytoplasmic dynein properties by phospho- and dephosphomimic mutations of the intermediate chain 2C S84
Abstract
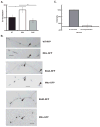
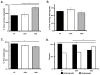
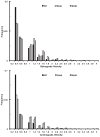
Cytoplasmic dynein is a multisubunit motor protein responsible for intracellular cargo transport toward microtubule minus ends. There are multiple isoforms of the dynein intermediate chain (DYNC1I, IC), which is encoded by two genes. One way to regulate cytoplasmic dynein is by IC phosphorylation. The IC-2C isoform is expressed in all cells, and the functional significance of phosphorylation on IC-2C serine 84 was investigated by using live cell imaging of fluorescent protein-tagged IC-2C wild type (WT) and phospho- and dephosphomimic mutant isoforms in axonal transport model systems. Both mutations modulated dynein functional properties. The dephosphomimic mutant IC-2C S84A had greater colocalization with mitochondria than the IC-2C WT or the phosphomimic mutant IC-2C S84D. The dephosphomimic mutant IC-2C S84A was also more likely to be motile than the phosphomimic mutant IC-2C S84D or the IC-2C WT. In contrast, the phosphomimic mutant IC-2C S84D mutant was more likely to move in the retrograde direction than was the IC-2C S84A mutant. The phosphomimic IC-2C S84D was also as likely as the IC-2C WT to colocalize with mitochondria. Both the S84D phospho- and the S84A dephosphomimic mutants were found to be capable of microtubule minus-end-directed (retrograde) movement in axons. They were also observed to be passively transported in the anterograde direction. These data suggest that the IC-2C S84 has a role in modulating dynein properties.
Keywords: axonal transport; cytoplasmic dynein; cytoskeleton; motor protein.
© 2014 Wiley Periodicals, Inc.
Figures

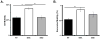
References
-
- Abe TK, Honda T, Takei K, Mikoshiba K, Hoffman-Kim D, Jay DG, Kuwano R. Dynactin is essential for growth cone advance. Biochemical and biophysical research communications. 2008;372(3):418–422. - PubMed
-
- Addinall SG, Mayr PS, Doyle S, Sheehan JK, Woodman PG, Allan VJ. Phosphorylation by cdc2-CyclinB1 kinase releases cytoplasmic dynein from membranes. The Journal of biological chemistry. 2001;276(19):15939–15944. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

