Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation
- PMID: 24798736
- PMCID: PMC4018789
- DOI: 10.1083/jcb.201309141
Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation
Abstract
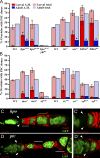
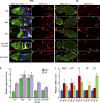
It is essential to define the mechanisms by which external signals regulate adult stem cell numbers, stem cell maintenance, and stem cell proliferation to guide regenerative stem cell therapies and to understand better how cancers originate in stem cells. In this paper, we show that Hedgehog (Hh) signaling in Drosophila melanogaster ovarian follicle stem cells (FSCs) induces the activity of Yorkie (Yki), the transcriptional coactivator of the Hippo pathway, by inducing yki transcription. Moreover, both Hh signaling and Yki positively regulate the rate of FSC proliferation, both are essential for FSC maintenance, and both promote increased FSC longevity and FSC duplication when in excess. We also found that responses to activated Yki depend on Cyclin E induction while responses to excess Hh signaling depend on Yki induction, and excess Yki can compensate for defective Hh signaling. These causal connections provide the most rigorous evidence to date that a niche signal can promote stem cell maintenance principally by stimulating stem cell proliferation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

