Deficiency in perlecan/HSPG2 during bone development enhances osteogenesis and decreases quality of adult bone in mice
- PMID: 24798737
- PMCID: PMC4137566
- DOI: 10.1007/s00223-014-9859-2
Deficiency in perlecan/HSPG2 during bone development enhances osteogenesis and decreases quality of adult bone in mice
Abstract
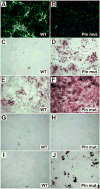
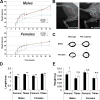
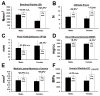
Perlecan/HSPG2 (Pln) is a large heparan sulfate proteoglycan abundant in the extracellular matrix of cartilage and the lacunocanalicular space of adult bones. Although Pln function during cartilage development is critical, evidenced by deficiency disorders including Schwartz-Jampel Syndrome and dyssegmental dysplasia Silverman-Handmaker type, little is known about its function in development of bone shape and quality. The purpose of this study was to understand the contribution of Pln to bone geometric and mechanical properties. We used hypomorph mutant mice that secrete negligible amount of Pln into skeletal tissues and analyzed their adult bone properties using micro-computed tomography and three-point-bending tests. Bone shortening and widening in Pln mutants was observed and could be attributed to loss of growth plate organization and accelerated osteogenesis that was reflected by elevated cortical thickness at older ages. This effect was more pronounced in Pln mutant females, indicating a sex-specific effect of Pln deficiency on bone geometry. Additionally, mutant females, and to a lesser extent mutant males, increased their elastic modulus and bone mineral densities to counteract changes in bone shape, but at the expense of increased brittleness. In summary, Pln deficiency alters cartilage matrix patterning and, as we now show, coordinately influences bone formation and calcification.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Trabecular Bone Deficit and Enhanced Anabolic Response to Re-Ambulation after Disuse in Perlecan-Deficient Skeleton.Biomolecules. 2020 Jan 29;10(2):198. doi: 10.3390/biom10020198. Biomolecules. 2020. PMID: 32013135 Free PMC article.
-
Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone.J Bone Miner Res. 2011 Mar;26(3):618-29. doi: 10.1002/jbmr.236. J Bone Miner Res. 2011. PMID: 20814969 Free PMC article.
-
Dyssegmental dysplasia, Silverman-Handmaker type: unexpected role of perlecan in cartilage development.Am J Med Genet. 2001 Winter;106(4):254-7. doi: 10.1002/ajmg.10229. Am J Med Genet. 2001. PMID: 11891676 Review.
-
Schwartz-Jampel syndrome and perlecan deficiency.Acta Myol. 2005 Oct;24(2):89-92. Acta Myol. 2005. PMID: 16550923 Review.
-
Impact of the heparan sulfate proteoglycan perlecan on human disease and health.Am J Physiol Cell Physiol. 2022 Jun 1;322(6):C1117-C1122. doi: 10.1152/ajpcell.00113.2022. Epub 2022 Apr 13. Am J Physiol Cell Physiol. 2022. PMID: 35417267 Review.
Cited by
-
Perlecan/Hspg2 deficiency impairs bone's calcium signaling and associated transcriptome in response to mechanical loading.Bone. 2020 Feb;131:115078. doi: 10.1016/j.bone.2019.115078. Epub 2019 Nov 9. Bone. 2020. PMID: 31715337 Free PMC article.
-
Extracellular matrix and developing growth plate.Curr Osteoporos Rep. 2014 Dec;12(4):439-45. doi: 10.1007/s11914-014-0232-1. Curr Osteoporos Rep. 2014. PMID: 25212565 Review.
-
Mucopolysaccharidosis Type I: A Review of the Natural History and Molecular Pathology.Cells. 2020 Aug 5;9(8):1838. doi: 10.3390/cells9081838. Cells. 2020. PMID: 32764324 Free PMC article. Review.
-
[Research progress in osteogenesis and osteogenic mechanism of heparan sulfate].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017 Aug 15;31(8):1016-1020. doi: 10.7507/1002-1892.201701103. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017. PMID: 29806444 Free PMC article. Review. Chinese.
-
FAM20B-Catalyzed Glycosylation Regulates the Chondrogenic and Osteogenic Differentiation of the Embryonic Condyle by Controlling IHH Diffusion and Release.Int J Mol Sci. 2025 Apr 24;26(9):4033. doi: 10.3390/ijms26094033. Int J Mol Sci. 2025. PMID: 40362273 Free PMC article.
References
-
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nature genetics. 1999;23:354–358. - PubMed
-
- Stum M, Davoine CS, Vicart S, Guillot-Noel L, Topaloglu H, Carod-Artal FJ, Kayserili H, Hentati F, Merlini L, Urtizberea JA, Hammouda el H, Quan PC, Fontaine B, Nicole S. Spectrum of HSPG2 (Perlecan) mutations in patients with Schwartz-Jampel syndrome. Human mutation. 2006;27:1082–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

