Synthesis, characterization and anti-breast cancer activity of new 4-aminoantipyrine-based heterocycles
- PMID: 24798749
- PMCID: PMC4057689
- DOI: 10.3390/ijms15057539
Synthesis, characterization and anti-breast cancer activity of new 4-aminoantipyrine-based heterocycles
Abstract
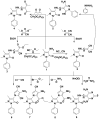
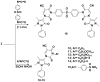
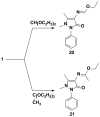
4-Aminoantipyrine was utilized as key intermediate for the synthesis of pyrazolone derivatives bearing biologically active moieties. The newly synthesized compounds were characterized by IR, 1H- and 13C-NMR spectral and microanalytical studies. The compounds were screened as anticancer agents against a human tumor breast cancer cell line MCF7, and the results showed that (Z)-4-((3-amino-5-imino-1-phenyl-1H-pyrazol-4(5H)-ylidene)methylamino)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one 5, 3-(4-bromophenyl) -1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 13, 1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-3-(4-iodophenyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 14, 3,3'-(4,4'-sulfonylbis(4,1-phenylene))bis(1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile) 16, (Z)-1- (1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-2-hydrazono-4-oxo-3-phenyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 17, (Z)-1-(1,5-dimethyl-3-oxo-2-phenyl- 2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-3-phenyl-2-(2-phenylhydrazono)-1,2,3,4-tetrahydro pyrimidine-5-carbonitrile 18, and (Z)-4-(3-amino-6-hydrazono-7-phenyl-6,7-dihydro pyrazolo[3,4-d]pyrimidin-5-yl)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one 19 were the most active compounds with IC50 values ranging from 30.68 to 60.72 µM compared with Doxorubicin as positive control with the IC50 value 71.8 µM.
Figures
Similar articles
-
A structural study of 4-aminoantipyrine and six of its Schiff base derivatives.Acta Crystallogr C Struct Chem. 2015 Feb;71(Pt 2):103-9. doi: 10.1107/S2053229614027247. Epub 2015 Jan 12. Acta Crystallogr C Struct Chem. 2015. PMID: 25652276
-
Non-steroidal antiinflammatory agents. Synthesis of novel 2-pyrazolyl-4(3H)-quinazolinones.Arch Pharm (Weinheim). 1990 Oct;323(10):833-6. doi: 10.1002/ardp.19903231004. Arch Pharm (Weinheim). 1990. PMID: 2080894
-
Synthesis, Characterization, Antimicrobial Activity and Anticancer of Some New Pyrazolo[1,5-a]pyrimidines and Pyrazolo[5,1-c]1,2,4-triazines.Med Chem. 2020;16(6):750-760. doi: 10.2174/1573406415666190620144404. Med Chem. 2020. PMID: 31218963
-
Pyrazole Containing Anti-HIV Agents: An Update.Med Chem. 2022;18(8):831-846. doi: 10.2174/1573406418666220106163846. Med Chem. 2022. PMID: 34994333 Review.
-
Apixaban.2023 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2023 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 29999800 Free Books & Documents. Review.
Cited by
-
BHX, a novel pyrazoline derivative, inhibits breast cancer cell invasion by reversing the epithelial-mesenchymal transition and down-regulating Wnt/β-catenin signalling.Sci Rep. 2017 Aug 22;7(1):9153. doi: 10.1038/s41598-017-09655-7. Sci Rep. 2017. PMID: 28831201 Free PMC article.
-
Synthesis and Cytotoxic Evaluation of Pyrrole Hetarylazoles Containing Benzimidazole/Pyrazolone/1,3,4-Oxadiazole Motifs.J Heterocycl Chem. 2016 Nov;53(6):1871-1877. doi: 10.1002/jhet.2501. Epub 2015 Sep 18. J Heterocycl Chem. 2016. PMID: 27956751 Free PMC article.
-
In-Vitro Anticancer Evaluation of Some Novel Thioureido-Benzensulfonamide Derivatives.Molecules. 2016 Mar 25;21(4):409. doi: 10.3390/molecules21040409. Molecules. 2016. PMID: 27023509 Free PMC article.
-
Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect.Eur J Med Chem. 2020 Jan 15;186:111893. doi: 10.1016/j.ejmech.2019.111893. Epub 2019 Nov 16. Eur J Med Chem. 2020. PMID: 31761383 Free PMC article. Review.
-
Synthesis, Antimicrobial and Antioxidant Activity of Pyrazole Based Sulfonamide Derivatives.Indian J Microbiol. 2018 Mar;58(1):93-99. doi: 10.1007/s12088-017-0689-6. Epub 2017 Oct 31. Indian J Microbiol. 2018. PMID: 29434402 Free PMC article.
References
-
- Himly M., Jahn-Schmid B., Pittertschatscher K., Bohle B., Grubmayr K., Ferreira F., Ebner H., Ebner C. Ig E-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone. J. Allergy Clin. Immunol. 2003;111:882–888. - PubMed
-
- Gürsoy A., Demirayak S., Capan G., Erol K., Vural K. Synthesis and preliminary evaluation of new 5-pyrazolinone derivatives as analgesic agents. Eur. J. Med. Chem. 2000;35:359–364. - PubMed
-
- Teng Y., Liu R., Li C., Zhang H. Effect of 4-aminoantipyrine on oxidative stress induced by glutathione depletion in single human erythrocytes using a microfluidic device together with fluorescence imaging. J. Hazard. Mater. 2011;192:1766–1771. - PubMed
-
- Turan-Zitouni G., Sivaci M., Kiliç F.S., Erol K. Synthesis of some triazolyl-antipyrine derivatives and investigation of analgesic activity. Eur. J. Med. Chem. 2001;36:685–689. - PubMed
-
- Lutsevich A.N., Bender K.I., Reshet’ko O.V. The relationship between antipyrine kinetics, the seromucoid content and the xanthine oxidase activity in the plasma of rats with acute and chronic inflammation. Eksp Klin. Farmakol. 1995;58:51–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous