Deciphering the metabolism of undecaprenyl-phosphate: the bacterial cell-wall unit carrier at the membrane frontier
- PMID: 24799078
- PMCID: PMC4050452
- DOI: 10.1089/mdr.2014.0035
Deciphering the metabolism of undecaprenyl-phosphate: the bacterial cell-wall unit carrier at the membrane frontier
Abstract
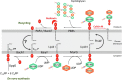
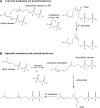
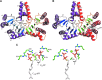
During the biogenesis of bacterial cell-wall polysaccharides, such as peptidoglycan, cytoplasmic synthesized precursors should be trafficked across the plasma membrane. This essential process requires a dedicated lipid, undecaprenyl-phosphate that is used as a glycan lipid carrier. The sugar is linked to the lipid carrier at the inner face of the membrane and is translocated toward the periplasm, where the glycan moiety is transferred to the growing polymer. Undecaprenyl-phosphate originates from the dephosphorylation of its precursor undecaprenyl-diphosphate, with itself generated by de novo synthesis or by recycling after the final glycan transfer. Undecaprenyl-diphosphate is de novo synthesized by the cytosolic cis-prenyltransferase undecaprenyl-diphosphate synthase, which has been structurally and mechanistically characterized in great detail highlighting the condensation process. In contrast, the next step toward the formation of the lipid carrier, the dephosphorylation step, which has been overlooked for many years, has only started revealing surprising features. In contrast to the previous step, two unrelated families of integral membrane proteins exhibit undecaprenyl-diphosphate phosphatase activity: BacA and members of the phosphatidic acid phosphatase type 2 super-family, raising the question of the significance of this multiplicity. Moreover, these enzymes establish an unexpected link between the synthesis of bacterial cell-wall polymers and other biological processes. In the present review, the current knowledge in the field of the bacterial lipid carrier, its mechanism of action, biogenesis, recycling, regulation, and future perspective works are presented.
Figures


References
-
- Barreteau H., Magnet S., El Ghachi M., Touzé T., Arthur M., Mengin-Lecreulx D., and Blanot D.2009. Quantitative high-performance liquid chromatography analysis of the pool levels of undecaprenyl phosphate and its derivatives in bacterial membranes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:213–220 - PubMed
-
- Bernard R., El Ghachi M., Mengin-Lecreulx D., Chippaux M., and Denizot F.2005. BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. J. Biol. Chem. 280:28852–28857 - PubMed
-
- Bernard R., Joseph P., Guiseppi A., Chippaux M., and Denizot F.2003. YtscD and YwoA, two independent systems that confer bacitracin resistance to Bacillus subtilis. FEMS Microbiol. Lett. 228:93–97 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
