Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus
- PMID: 24799499
- PMCID: PMC4042635
- DOI: 10.1084/jem.20131853
Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus
Abstract
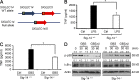
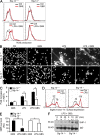
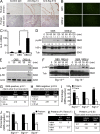
Group B Streptococcus (GBS) causes invasive infections in human newborns. We recently showed that the GBS β-protein attenuates innate immune responses by binding to sialic acid-binding immunoglobulin-like lectin 5 (Siglec-5), an inhibitory receptor on phagocytes. Interestingly, neutrophils and monocytes also express Siglec-14, which has a ligand-binding domain almost identical to Siglec-5 but signals via an activating motif, raising the possibility that these are paired Siglec receptors that balance immune responses to pathogens. Here we show that β-protein-expressing GBS binds to both Siglec-5 and Siglec-14 on neutrophils and that the latter engagement counteracts pathogen-induced host immune suppression by activating p38 mitogen-activated protein kinase (MAPK) and AKT signaling pathways. Siglec-14 is absent from some humans because of a SIGLEC14-null polymorphism, and homozygous SIGLEC14-null neutrophils are more susceptible to GBS immune subversion. Finally, we report an unexpected human-specific expression of Siglec-5 and Siglec-14 on amniotic epithelium, the site of initial contact of invading GBS with the fetus. GBS amnion immune activation was likewise influenced by the SIGLEC14-null polymorphism. We provide initial evidence that the polymorphism could influence the risk of prematurity among human fetuses of mothers colonized with GBS. This first functionally proven example of a paired receptor system in the Siglec family has multiple implications for regulation of host immunity.
© 2014 Ali et al.
Figures


Similar articles
-
The SIGLEC14 null allele is associated with Mycobacterium tuberculosis- and BCG-induced clinical and immunologic outcomes.Tuberculosis (Edinb). 2017 May;104:38-45. doi: 10.1016/j.tube.2017.02.005. Epub 2017 Feb 21. Tuberculosis (Edinb). 2017. PMID: 28454648 Free PMC article.
-
Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5.J Exp Med. 2009 Aug 3;206(8):1691-9. doi: 10.1084/jem.20090691. Epub 2009 Jul 13. J Exp Med. 2009. PMID: 19596804 Free PMC article.
-
Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec-5 and Siglec-14.EMBO J. 2015 Nov 12;34(22):2775-88. doi: 10.15252/embj.201591407. Epub 2015 Oct 12. EMBO J. 2015. PMID: 26459514 Free PMC article.
-
Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation?Immunology. 2011 Jan;132(1):18-26. doi: 10.1111/j.1365-2567.2010.03368.x. Epub 2010 Nov 11. Immunology. 2011. PMID: 21070233 Free PMC article. Review.
-
Viewing Siglecs through the lens of tumor immunology.Immunol Rev. 2017 Mar;276(1):178-191. doi: 10.1111/imr.12526. Immunol Rev. 2017. PMID: 28258691 Free PMC article. Review.
Cited by
-
Siglecs in Brain Function and Neurological Disorders.Cells. 2019 Sep 22;8(10):1125. doi: 10.3390/cells8101125. Cells. 2019. PMID: 31546700 Free PMC article. Review.
-
The interplay between Siglecs and sialylated pathogens.Glycobiology. 2014 Sep;24(9):818-25. doi: 10.1093/glycob/cwu067. Epub 2014 Jul 4. Glycobiology. 2014. PMID: 24996821 Free PMC article. Review.
-
Siglecs take a TOLL on inflammation: deciphering the Hsp70 riddle.EMBO J. 2015 Nov 12;34(22):2733-4. doi: 10.15252/embj.201593172. Epub 2015 Oct 16. EMBO J. 2015. PMID: 26474574 Free PMC article.
-
P-Selectin Glycoprotein Ligand 1: A Potential HIV-1 Therapeutic Target.Front Immunol. 2021 Aug 9;12:710121. doi: 10.3389/fimmu.2021.710121. eCollection 2021. Front Immunol. 2021. PMID: 34434194 Free PMC article. Review.
-
Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes.Sci Rep. 2017 Apr 5;7:45319. doi: 10.1038/srep45319. Sci Rep. 2017. PMID: 28378743 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

