Fanca deficiency reduces A/T transitions in somatic hypermutation and alters class switch recombination junctions in mouse B cells
- PMID: 24799500
- PMCID: PMC4042646
- DOI: 10.1084/jem.20131637
Fanca deficiency reduces A/T transitions in somatic hypermutation and alters class switch recombination junctions in mouse B cells
Abstract
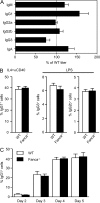
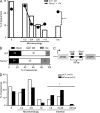
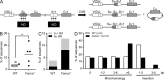
Fanconi anemia is a rare genetic disorder that can lead to bone marrow failure, congenital abnormalities, and increased risk for leukemia and cancer. Cells with loss-of-function mutations in the FANC pathway are characterized by chromosome fragility, altered mutability, and abnormal regulation of the nonhomologous end-joining (NHEJ) pathway. Somatic hypermutation (SHM) and immunoglobulin (Ig) class switch recombination (CSR) enable B cells to produce high-affinity antibodies of various isotypes. Both processes are initiated after the generation of dG:dU mismatches by activation-induced cytidine deaminase. Whereas SHM involves an error-prone repair process that introduces novel point mutations into the Ig gene, the mismatches generated during CSR are processed to create double-stranded breaks (DSBs) in DNA, which are then repaired by the NHEJ pathway. As several lines of evidence suggest a possible role for the FANC pathway in SHM and CSR, we analyzed both processes in B cells derived from Fanca(-/-) mice. Here we show that Fanca is required for the induction of transition mutations at A/T residues during SHM and that despite globally normal CSR function in splenic B cells, Fanca is required during CSR to stabilize duplexes between pairs of short microhomology regions, thereby impeding short-range recombination downstream of DSB formation.
© 2014 Nguyen et al.
Figures

Similar articles
-
DNA polymerases β and λ do not directly affect Ig variable region somatic hypermutation although their absence reduces the frequency of mutations.DNA Repair (Amst). 2013 Dec;12(12):1087-93. doi: 10.1016/j.dnarep.2013.09.002. Epub 2013 Sep 29. DNA Repair (Amst). 2013. PMID: 24084171 Free PMC article.
-
The ATPase activity of MLH1 is required to orchestrate DNA double-strand breaks and end processing during class switch recombination.J Exp Med. 2012 Apr 9;209(4):671-8. doi: 10.1084/jem.20111531. Epub 2012 Mar 26. J Exp Med. 2012. PMID: 22451719 Free PMC article.
-
A primary immunodeficiency characterized by defective immunoglobulin class switch recombination and impaired DNA repair.J Exp Med. 2007 May 14;204(5):1207-16. doi: 10.1084/jem.20070087. Epub 2007 May 7. J Exp Med. 2007. PMID: 17485519 Free PMC article.
-
Current insights into the mechanism of mammalian immunoglobulin class switch recombination.Crit Rev Biochem Mol Biol. 2019 Aug;54(4):333-351. doi: 10.1080/10409238.2019.1659227. Epub 2019 Sep 11. Crit Rev Biochem Mol Biol. 2019. PMID: 31509023 Free PMC article. Review.
-
Evolution of the immunoglobulin heavy chain class switch recombination mechanism.Adv Immunol. 2007;94:157-214. doi: 10.1016/S0065-2776(06)94006-1. Adv Immunol. 2007. PMID: 17560275 Review.
Cited by
-
V(D)J recombination process and the Pre-B to immature B-cells transition are altered in Fanca-/- mice.Sci Rep. 2016 Nov 24;6:36906. doi: 10.1038/srep36906. Sci Rep. 2016. PMID: 27883081 Free PMC article.
-
The Fanconi anaemia pathway: new players and new functions.Nat Rev Mol Cell Biol. 2016 Jun;17(6):337-49. doi: 10.1038/nrm.2016.48. Epub 2016 May 5. Nat Rev Mol Cell Biol. 2016. PMID: 27145721 Review.
-
The FANC/BRCA Pathway Releases Replication Blockades by Eliminating DNA Interstrand Cross-Links.Genes (Basel). 2020 May 25;11(5):585. doi: 10.3390/genes11050585. Genes (Basel). 2020. PMID: 32466131 Free PMC article. Review.
-
Emerging functions of the Fanconi anemia pathway at a glance.J Cell Sci. 2017 Aug 15;130(16):2657-2662. doi: 10.1242/jcs.204909. J Cell Sci. 2017. PMID: 28811338 Free PMC article. Review.
-
Loss of Fancc Impairs Antibody-Secreting Cell Differentiation in Mice through Deregulating the Wnt Signaling Pathway.J Immunol. 2016 Apr 1;196(7):2986-94. doi: 10.4049/jimmunol.1501056. Epub 2016 Feb 19. J Immunol. 2016. PMID: 26895835 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

