IKKβ links vascular inflammation to obesity and atherosclerosis
- PMID: 24799533
- PMCID: PMC4010900
- DOI: 10.1084/jem.20131281
IKKβ links vascular inflammation to obesity and atherosclerosis
Abstract
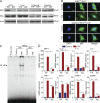
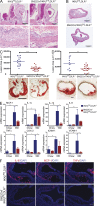
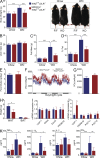
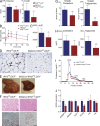
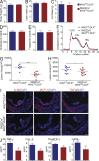
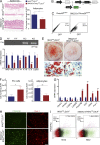
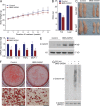
IκB kinase β (IKKβ), a central coordinator of inflammatory responses through activation of NF-κB, has been implicated in vascular pathologies, but its role in atherogenesis remains elusive. Here, we demonstrate that IKKβ functions in smooth muscle cells (SMCs) to regulate vascular inflammatory responses and atherosclerosis development. IKKβ deficiency in SMCs driven by a SM22Cre-IKKβ-flox system rendered low density lipoprotein receptor-null mice resistant to vascular inflammation and atherosclerosis induced by high-fat feeding. Unexpectedly, IKKβ-deficient mice were also resistant to diet-induced obesity and metabolic disorders. Cell lineage analysis revealed that SM22Cre is active in primary adipose stromal vascular cells and deficiency of IKKβ diminished the ability of these cells to differentiate, leading to accumulation of adipocyte precursor cells in adipose tissue. Mechanistically, reduction of IKKβ expression or pharmacological inhibition of IKKβ inhibited proteasome-mediated β-catenin ubiquitination and degradation in murine preadipocytes, resulting in elevated β-catenin levels and impaired adipocyte differentiation. Further, chronic treatment of mice with a potent IKKβ inhibitor decreased adipogenesis and ameliorated diet-induced obesity. Our findings demonstrate a pivotal role of IKKβ in linking vascular inflammation to atherosclerosis and adipose tissue development, and provide evidence for using appropriate IKKβ inhibitors in the treatment of obesity and metabolic disorders.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

