Simian virus 40 large T antigen induces IFN-stimulated genes through ATR kinase
- PMID: 24799566
- PMCID: PMC4078001
- DOI: 10.4049/jimmunol.1303470
Simian virus 40 large T antigen induces IFN-stimulated genes through ATR kinase
Abstract
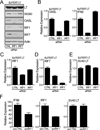
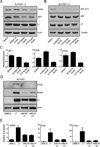
Polyomaviruses encode a large T Ag (LT), a multifunctional protein essential for the regulation of both viral and host cell gene expression and productive viral infection. Previously, we have shown that stable expression of LT protein results in upregulation of genes involved in the IFN induction and signaling pathway. In this study, we focus on the cellular signaling mechanism that leads to the induction of IFN responses by LT. Our results show that ectopic expression of SV40 LT results in the induction of IFN-stimulated genes (ISGs) in human fibroblasts and confers an antiviral state. We describe a LT-initiated DNA damage response (DDR) that activates IFN regulatory factor 1, causing IFN-β production and consequent ISG expression in human cells. This IFN-β and ISG induction is dependent on ataxia-telangiectasia mutated and Rad3-related (ATR) kinase, but independent of ATM. ATR kinase inhibition using a selective kinase inhibitor (ETP-46464) caused a decrease in IFN regulatory factor 1 stabilization and ISG expression. Furthermore, expression of a mutant LT that does not induce DDR also does not induce IFN-β and ISGs. These results show that, in the absence of viral infection, LT-initiated activation of ATR-dependent DDR is sufficient for the induction of an IFN-β-mediated innate immune response in human cells. Thus, we have uncovered a novel and critical role for ATR as a mediator of antiviral responses utilizing LT.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding.J Virol. 2009 Jan;83(1):117-27. doi: 10.1128/JVI.01515-08. Epub 2008 Oct 15. J Virol. 2009. PMID: 18922873 Free PMC article.
-
Viral DNA replication-dependent DNA damage response activation during BK polyomavirus infection.J Virol. 2015 May;89(9):5032-9. doi: 10.1128/JVI.03650-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694603 Free PMC article.
-
Simian virus 40 activates ATR-Delta p53 signaling to override cell cycle and DNA replication control.J Virol. 2010 Oct;84(20):10727-47. doi: 10.1128/JVI.00122-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686026 Free PMC article.
-
The development of ataxia telangiectasia mutated kinase inhibitors.Mini Rev Med Chem. 2014;14(10):805-11. Mini Rev Med Chem. 2014. PMID: 25138084 Review.
-
Polyomavirus interaction with the DNA damage response.Virol Sin. 2015 Apr;30(2):122-9. doi: 10.1007/s12250-015-3583-6. Epub 2015 Apr 20. Virol Sin. 2015. PMID: 25910481 Free PMC article. Review.
Cited by
-
Transcriptome sequencing wide functional analysis of human mesenchymal stem cells in response to TLR4 ligand.Sci Rep. 2016 Jul 22;6:30311. doi: 10.1038/srep30311. Sci Rep. 2016. PMID: 27444640 Free PMC article.
-
MEF2A suppresses stress responses that trigger DDX41-dependent IFN production.Cell Rep. 2023 Aug 29;42(8):112805. doi: 10.1016/j.celrep.2023.112805. Epub 2023 Jul 18. Cell Rep. 2023. PMID: 37467105 Free PMC article.
-
Checkpoint inhibitor blockade and epigenetic reprogrammability in CD8+ T-cell activation and exhaustion.Ther Adv Vaccines Immunother. 2020 Mar 13;8:2515135520904238. doi: 10.1177/2515135520904238. eCollection 2020. Ther Adv Vaccines Immunother. 2020. PMID: 32206744 Free PMC article. Review.
-
Transcriptome sequencing reveals that LPS-triggered transcriptional responses in established microglia BV2 cell lines are poorly representative of primary microglia.J Neuroinflammation. 2016 Jul 11;13(1):182. doi: 10.1186/s12974-016-0644-1. J Neuroinflammation. 2016. PMID: 27400875 Free PMC article.
-
Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide.J Endocrinol. 2019 Feb 1;240(2):243-256. doi: 10.1530/JOE-18-0370. J Endocrinol. 2019. PMID: 30530902 Free PMC article.
References
-
- Zurhein G, Chou SM. Particles Resembling Papova Viruses in Human Cerebral Demyelinating Disease. Science. 1965;148:1477–1479. - PubMed
-
- Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–1260. - PubMed
-
- Pipas JM. SV40: Cell transformation and tumorigenesis. Virology. 2009;384:294–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

