Angiotensin 1-7 reduces mortality and rupture of intracranial aneurysms in mice
- PMID: 24799613
- PMCID: PMC4096422
- DOI: 10.1161/HYPERTENSIONAHA.114.03415
Angiotensin 1-7 reduces mortality and rupture of intracranial aneurysms in mice
Abstract
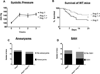
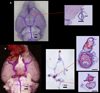
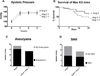
Angiotensin II (Ang II) stimulates vascular inflammation, oxidative stress, and formation and rupture of intracranial aneurysms in mice. Because Ang 1-7 acts on Mas receptors and generally counteracts deleterious effects of Ang II, we tested the hypothesis that Ang 1-7 attenuates formation and rupture of intracranial aneurysms. Intracranial aneurysms were induced in wild-type and Mas receptor-deficient mice using a combination of Ang II-induced hypertension and intracranial injection of elastase in the basal cistern. Mice received elastase+Ang II alone or a combination of elastase+Ang II+Ang 1-7. Aneurysm formation, prevalence of subarachnoid hemorrhage, mortality, and expression of molecules involved in vascular injury were assessed. Systolic blood pressure was similar in mice receiving elastase+Ang II (mean±SE, 148±5 mm Hg) or elastase+Ang II+Ang 1-7 (144±5 mm Hg). Aneurysm formation was also similar in mice receiving elastase+Ang II (89%) or elastase+Ang II+Ang 1-7 (84%). However, mice that received elastase+Ang II+Ang 1-7 had reduced mortality (from 64% to 36%; P<0.05) and prevalence of subarachnoid hemorrhage (from 75% to 48%; P<0.05). In cerebral arteries, expression of the inflammatory markers, Nox2 and catalase increased similarly in elastase+Ang II or elastase+Ang II+Ang 1-7 groups. Ang 1-7 increased the expression of cyclooxygenase-2 and decreased the expression of matrix metalloproteinase-9 induced by elastase+Ang II (P<0.05). In Mas receptor-deficient mice, systolic blood pressure, mortality, and prevalence of subarachnoid hemorrhage were similar (P>0.05) in groups treated with elastase+Ang II or elastase+Ang II+Ang 1-7. The expression of Mas receptor was detected by immunohistochemistry in samples of human intracranial arteries and aneurysms. In conclusion, without attenuating Ang II-induced hypertension, Ang 1-7 decreased mortality and rupture of intracranial aneurysms in mice through a Mas receptor-dependent pathway.
Keywords: angiotein (1–7); angiotensin (1–7) receptor Mas, human; hypertension; intracranial aneurysm; subarachnoid hemorrhage.
© 2014 American Heart Association, Inc.
Figures



Comment in
-
Angiotensin (1-7) as a therapy to prevent rupture of intracranial aneurysms?Hypertension. 2014 Aug;64(2):222-3. doi: 10.1161/HYPERTENSIONAHA.114.03551. Hypertension. 2014. PMID: 24799616 No abstract available.
References
-
- Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

