Bile acids and bicarbonate inversely regulate intracellular cyclic di-GMP in Vibrio cholerae
- PMID: 24799624
- PMCID: PMC4097643
- DOI: 10.1128/IAI.01664-14
Bile acids and bicarbonate inversely regulate intracellular cyclic di-GMP in Vibrio cholerae
Abstract
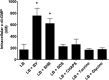
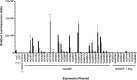
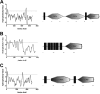
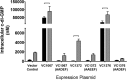
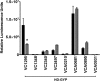
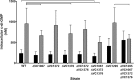
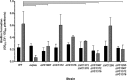
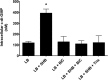
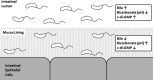
Vibrio cholerae is a Gram-negative bacterium that persists in aquatic reservoirs and causes the diarrheal disease cholera upon entry into a human host. V. cholerae employs the second messenger molecule 3',5'-cyclic diguanylic acid (c-di-GMP) to transition between these two distinct lifestyles. c-di-GMP is synthesized by diguanylate cyclase (DGC) enzymes and hydrolyzed by phosphodiesterase (PDE) enzymes. Bacteria typically encode many different DGCs and PDEs within their genomes. Presumably, each enzyme senses and responds to cognate environmental cues by alteration of enzymatic activity. c-di-GMP represses the expression of virulence factors in V. cholerae, and it is predicted that the intracellular concentration of c-di-GMP is low during infection. Contrary to this model, we found that bile acids, a prevalent constituent of the human proximal small intestine, increase intracellular c-di-GMP in V. cholerae. We identified four c-di-GMP turnover enzymes that contribute to increased intracellular c-di-GMP in the presence of bile acids, and deletion of these enzymes eliminates the bile induction of c-di-GMP and biofilm formation. Furthermore, this bile-mediated increase in c-di-GMP is quenched by bicarbonate, the intestinal pH buffer secreted by intestinal epithelial cells. Our results lead us to propose that V. cholerae senses distinct microenvironments within the small intestine using bile and bicarbonate as chemical cues and responds by modulating the intracellular concentration of c-di-GMP.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

