Coevolution can reverse predator-prey cycles
- PMID: 24799689
- PMCID: PMC4034221
- DOI: 10.1073/pnas.1317693111
Coevolution can reverse predator-prey cycles
Abstract
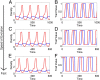
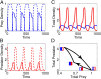
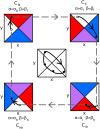
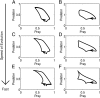
A hallmark of Lotka-Volterra models, and other ecological models of predator-prey interactions, is that in predator-prey cycles, peaks in prey abundance precede peaks in predator abundance. Such models typically assume that species life history traits are fixed over ecologically relevant time scales. However, the coevolution of predator and prey traits has been shown to alter the community dynamics of natural systems, leading to novel dynamics including antiphase and cryptic cycles. Here, using an eco-coevolutionary model, we show that predator-prey coevolution can also drive population cycles where the opposite of canonical Lotka-Volterra oscillations occurs: predator peaks precede prey peaks. These reversed cycles arise when selection favors extreme phenotypes, predator offense is costly, and prey defense is effective against low-offense predators. We present multiple datasets from phage-cholera, mink-muskrat, and gyrfalcon-rock ptarmigan systems that exhibit reversed-peak ordering. Our results suggest that such cycles are a potential signature of predator-prey coevolution and reveal unique ways in which predator-prey coevolution can shape, and possibly reverse, community dynamics.
Keywords: community ecology; eco-coevolutionary dynamics; fast–slow dynamics; population biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Elton CS. Periodic fluctuations in the number of animals: Their causes and effects. J Exp Biol. 1924;2:119–163.
-
- Gause GF. Experimental demonstration of Volterra’s periodic oscillations in the numbers of animals. J Exp Biol. 1935;12(1):44–48.
-
- Elton CS, Nicholson M. Fluctuations in numbers of the muskrat (Ondatra zibethica) in Canada. J Anim Ecol. 1942;11(1):96–126.
-
- Rosenzweig ML, MacArthur RH. Graphical representation and stability conditions of predator-prey interactions. Am Nat. 1963;97(895):209–223.
-
- Lotka AJ. 1934. Théorie analytique des associations biologiques, Hermann, Paris.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

