Rich-club organization of the newborn human brain
- PMID: 24799693
- PMCID: PMC4034228
- DOI: 10.1073/pnas.1324118111
Rich-club organization of the newborn human brain
Abstract
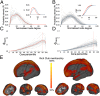
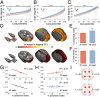
Combining diffusion magnetic resonance imaging and network analysis in the adult human brain has identified a set of highly connected cortical hubs that form a "rich club"--a high-cost, high-capacity backbone thought to enable efficient network communication. Rich-club architecture appears to be a persistent feature of the mature mammalian brain, but it is not known when this structure emerges during human development. In this longitudinal study we chart the emergence of structural organization in mid to late gestation. We demonstrate that a rich club of interconnected cortical hubs is already present by 30 wk gestation. Subsequently, until the time of normal birth, the principal development is a proliferation of connections between core hubs and the rest of the brain. We also consider the impact of environmental factors on early network development, and compare term-born neonates to preterm infants at term-equivalent age. Though rich-club organization remains intact following premature birth, we reveal significant disruptions in both in cortical-subcortical connectivity and short-distance corticocortical connections. Rich club organization is present well before the normal time of birth and may provide the fundamental structural architecture for the subsequent emergence of complex neurological functions. Premature exposure to the extrauterine environment is associated with altered network architecture and reduced network capacity, which may in part account for the high prevalence of cognitive problems in preterm infants.
Keywords: brain development; connectome; preterm birth; tractography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

