Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling
- PMID: 24799700
- PMCID: PMC4034241
- DOI: 10.1073/pnas.1403252111
Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling
Abstract
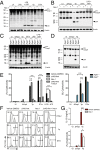
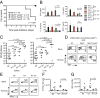
Toll-like receptor signaling and subsequent activation of NF-κB- and MAPK-dependent genes during infection play an important role in antimicrobial host defense. The YopJ protein of pathogenic Yersinia species inhibits NF-κB and MAPK signaling, resulting in blockade of NF-κB-dependent cytokine production and target cell death. Nevertheless, Yersinia infection induces inflammatory responses in vivo. Moreover, increasing the extent of YopJ-dependent cytotoxicity induced by Yersinia pestis and Yersinia pseudotuberculosis paradoxically leads to decreased virulence in vivo, suggesting that cell death promotes anti-Yersinia host defense. However, the specific pathways responsible for YopJ-induced cell death and how this cell death mediates immune defense against Yersinia remain poorly defined. YopJ activity induces processing of multiple caspases, including caspase-1, independently of inflammasome components or the adaptor protein ASC. Unexpectedly, caspase-1 activation in response to the activity of YopJ required caspase-8, receptor-interacting serine/threonine kinase 1 (RIPK1), and Fas-associated death domain (FADD), but not RIPK3. Furthermore, whereas RIPK3 deficiency did not affect YopJ-induced cell death or caspase-1 activation, deficiency of both RIPK3 and caspase-8 or FADD completely abrogated Yersinia-induced cell death and caspase-1 activation. Mice lacking RIPK3 and caspase-8 in their hematopoietic compartment showed extreme susceptibility to Yersinia and were deficient in monocyte and neutrophil-derived production of proinflammatory cytokines. Our data demonstrate for the first time to our knowledge that RIPK1, FADD, and caspase-8 are required for YopJ-induced cell death and caspase-1 activation and suggest that caspase-8-mediated cell death overrides blockade of immune signaling by YopJ to promote anti-Yersinia immune defense.
Keywords: apoptosis; innate immunity; macrophage; programmed necrosis; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. - PubMed
-
- Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13(8):551–565. - PubMed
-
- Mukherjee S, et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312(5777):1211–1214. - PubMed
-
- Orth K, et al. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 1999;285(5435):1920–1923. - PubMed
-
- Palmer LE, Hobbie S, Galán JE, Bliska JB. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27(5):953–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

