Basal NF-κB controls IL-7 responsiveness of quiescent naïve T cells
- PMID: 24799710
- PMCID: PMC4034246
- DOI: 10.1073/pnas.1315398111
Basal NF-κB controls IL-7 responsiveness of quiescent naïve T cells
Abstract
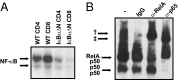
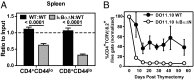
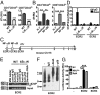
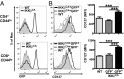
T cells are essential for immune defenses against pathogens, such that viability of naïve T cells before antigen encounter is critical to preserve a polyclonal repertoire and prevent immunodeficiencies. The viability of naïve T cells before antigen recognition is ensured by IL-7, which drives expression of the prosurvival factor Bcl-2. Quiescent naïve T cells have low basal activity of the transcription factor NF-κB, which was assumed to have no functional consequences. In contrast to this postulate, our data show that basal nuclear NF-κB activity plays an important role in the transcription of IL-7 receptor α-subunit (CD127), enabling responsiveness of naïve T cells to the prosurvival effects of IL-7 and allowing T-cell persistence in vivo. Moreover, we show that this property of basal NF-κB activity is shared by mouse and human naïve T cells. Thus, NF-κB drives a distinct transcriptional program in T cells before antigen encounter by controlling susceptibility to IL-7. Our results reveal an evolutionarily conserved role of NF-κB in T cells before antigenic stimulation and identify a novel molecular pathway that controls T-cell homeostasis.
Keywords: IKKβ; IkBaDN; Il7r enhancer; STAT5.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Enhanced interaction between SEC2 mutant and TCR Vβ induces MHC II-independent activation of T cells via PKCθ/NF-κB and IL-2R/STAT5 signaling pathways.J Biol Chem. 2018 Dec 21;293(51):19771-19784. doi: 10.1074/jbc.RA118.003668. Epub 2018 Oct 23. J Biol Chem. 2018. PMID: 30352872 Free PMC article.
-
Notch3 and pre-TCR interaction unveils distinct NF-kappaB pathways in T-cell development and leukemia.EMBO J. 2006 Mar 8;25(5):1000-8. doi: 10.1038/sj.emboj.7600996. Epub 2006 Feb 23. EMBO J. 2006. PMID: 16498412 Free PMC article.
-
IL-7-dependent STAT-5 activation and CD8+ T cell proliferation are impaired in HIV infection.J Leukoc Biol. 2011 Apr;89(4):499-506. doi: 10.1189/jlb.0710430. Epub 2010 Dec 21. J Leukoc Biol. 2011. PMID: 21177484
-
NFKB1: a suppressor of inflammation, ageing and cancer.FEBS J. 2016 May;283(10):1812-22. doi: 10.1111/febs.13627. Epub 2016 Jan 13. FEBS J. 2016. PMID: 26663363 Review.
-
IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis.Semin Immunol. 2012 Jun;24(3):209-17. doi: 10.1016/j.smim.2012.04.010. Epub 2012 May 1. Semin Immunol. 2012. PMID: 22551764 Free PMC article. Review.
Cited by
-
Tolerance and Cross-Tolerance following Toll-Like Receptor (TLR)-4 and -9 Activation Are Mediated by IRAK-M and Modulated by IL-7 in Murine Splenocytes.PLoS One. 2015 Jul 28;10(7):e0132921. doi: 10.1371/journal.pone.0132921. eCollection 2015. PLoS One. 2015. PMID: 26218271 Free PMC article.
-
Integrative understanding of immune-metabolic interaction.BMB Rep. 2022 Jun;55(6):259-266. doi: 10.5483/BMBRep.2022.55.6.064. BMB Rep. 2022. PMID: 35651325 Free PMC article. Review.
-
Transvitreal Retinochoroidal Biopsies of Primary Uveal Melanoma Reveal an Association of Low HLA Class I and High NK Cell Abundance in Low-Risk Disease.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):24. doi: 10.1167/iovs.66.2.24. Invest Ophthalmol Vis Sci. 2025. PMID: 39918475 Free PMC article.
-
TNF activation of NF-κB is essential for development of single-positive thymocytes.J Exp Med. 2016 Jul 25;213(8):1399-407. doi: 10.1084/jem.20151604. Epub 2016 Jul 18. J Exp Med. 2016. PMID: 27432943 Free PMC article.
-
Identification of a Novel Nonsense Mutation in NFKB1 Causing Common Variable Immunodeficiency with Decreased Tfh Cells.J Clin Immunol. 2023 Nov;43(8):1784-1787. doi: 10.1007/s10875-023-01588-3. Epub 2023 Sep 30. J Clin Immunol. 2023. PMID: 37775675 No abstract available.
References
-
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. - PubMed
-
- Murali-Krishna K, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286(5443):1377–1381. - PubMed
-
- Markiewicz MA, Brown I, Gajewski TF. Death of peripheral CD8+ T cells in the absence of MHC class I is Fas-dependent and not blocked by Bcl-xL. Eur J Immunol. 2003;33(10):2917–2926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

