Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome
- PMID: 24799711
- PMCID: PMC4034187
- DOI: 10.1073/pnas.1401657111
Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome
Erratum in
-
Correction for Kaushal et al., Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome.Proc Natl Acad Sci U S A. 2015 May 19;112(20):E2736. doi: 10.1073/pnas.1507224112. Epub 2015 Apr 28. Proc Natl Acad Sci U S A. 2015. PMID: 25922530 Free PMC article. No abstract available.
Abstract
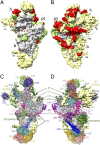
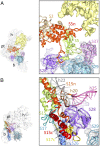
The mammalian mitochondrial ribosomes (mitoribosomes) are responsible for synthesizing 13 membrane proteins that form essential components of the complexes involved in oxidative phosphorylation or ATP generation for the eukaryotic cell. The mammalian 55S mitoribosome contains significantly smaller rRNAs and a large mass of mitochondrial ribosomal proteins (MRPs), including large mito-specific amino acid extensions and insertions in MRPs that are homologous to bacterial ribosomal proteins and an additional 35 mito-specific MRPs. Here we present the cryo-EM structure analysis of the small (28S) subunit (SSU) of the 55S mitoribosome. We find that the mito-specific extensions in homologous MRPs generally are involved in inter-MRP contacts and in contacts with mito-specific MRPs, suggesting a stepwise evolution of the current architecture of the mitoribosome. Although most of the mito-specific MRPs and extensions of homologous MRPs are situated on the peripheral regions, they also contribute significantly to the formation of linings of the mRNA and tRNA paths, suggesting a tailor-made structural organization of the mito-SSU for the recruitment of mito-specific mRNAs, most of which do not possess a 5' leader sequence. In addition, docking of previously published coordinates of the large (39S) subunit (LSU) into the cryo-EM map of the 55S mitoribosome reveals that mito-specific MRPs of both the SSU and LSU are involved directly in the formation of six of the 15 intersubunit bridges.
Keywords: cryo-electron microscopy; mammalian MRPs; mammalian mitochondrial ribosomal SSU; mito-12S rRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- O’Brien TW, O’Brien BJ, Norman RA. Nuclear MRP genes and mitochondrial disease. Gene. 2005;354:147–151. - PubMed
-
- Pearce S, Nezich CL, Spinazzola A. Mitochondrial diseases: Translation matters. Mol Cell Neurosci. 2013;55:1–12. - PubMed
-
- Sharma MR, et al. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115(1):97–108. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

