Effects of ascorbic acid on sperm motility, viability, acrosome reaction and DNA integrity in teratozoospermic samples
- PMID: 24799867
- PMCID: PMC4009562
Effects of ascorbic acid on sperm motility, viability, acrosome reaction and DNA integrity in teratozoospermic samples
Abstract
Background: Oxidative stress in teratozoospermic semen samples caused poor assisted reproductive techniques (ART) outcomes. Among antioxidants, ascorbic acid is a naturally occurring free radical scavenger and as such its presence assists various other mechanisms in decreasing numerous disruptive free radical processes.
Objective: The main goal of this study was to evaluate potential protective effects of ascorbic acid supplementation during in vitro culture of teratozoospermic specimens.
Materials and methods: Teratozoospermic semen samples that collected from 15 volunteers were processed, centrifuged and incubated at 37(o)C until sperm swimmed-up. Supernatant was divided into four groups and incubated at 37(o)C for one hour under different experimental conditions: Control, 10 µm A23187, 600µm ascorbic acid and 10 µm A23187+600 µm ascorbic acid. After incubation sperm motility, viability, acrosome reaction, DNA damage and malondialdehyde levels were evaluated.
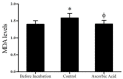
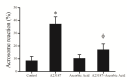
Results: Our results indicated that after one hour incubation, ascorbic acid significantly reduced malondialdehyde level in ascorbic acid group (1.4±0.11 nmol/ml) compared to control group (1.58±0.13 nmol/ml) (p<0.001). At the end of incubation, progressive motility and viability in ascorbic acid group (64.5±8.8% and 80.3±6.4%, respectively) were significantly (p<0.05 and p<0.001, respectively) higher than the control group (54.5±6.8% and 70.9±7.3%, respectively). A23187 significantly (p<0.0001) increased acrosome reaction in A23187 group (37.3±5.6%) compared to control group (8.5±3.2%) and this effect of A23187 attenuated by ascorbic acid in ascorbic acid+A23187 group (17.2±4.4%). DNA fragmentation in ascorbic acid group (20±4.1%) was significantly (p<0.001) lower than controls (28.9±4.6%).
Conclusion: In vitro ascorbic acid supplementation during teratozoospermic semen processing for ART could protect teratozoospermic specimens against oxidative stress, and it could improve ART outcome.
Keywords: Acrosome reaction; Ascorbic acid; DNA fragmentation; Oxidative stress; Teratozoospermic sperm.
Figures

References
-
- Keshtgar S, Fanaei H, Bahmanpour S, Azad F, Ghannadi A, Kazeroni M. In vitro effects of alpha-tocopherol on teratozoospermic semen samples. Andrologia. 2012;44 (Suppl.):721–727. - PubMed
-
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. - PubMed
-
- Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95:503–507. - PubMed
-
- Brahem S, Elghezal H, Ghedir H, Landolsi H, Amara A, Ibala S, et al. Cytogenetic and molecular aspects of absolute teratozoospermia: comparison between polymorphic and monomorphic forms. Urology. 2011;78:1313–1319. - PubMed
-
- Lu JC, Huang YF, Lu NQ. [WHO Laboratory Manual for the Examination and Processing of Human Semen: its applicability to andrology laboratories in China] Zhonghua Nan Ke Xue. 2010;16:867–871. - PubMed
LinkOut - more resources
Full Text Sources
