Effects of stingless bee propolis on experimental asthma
- PMID: 24799946
- PMCID: PMC3995315
- DOI: 10.1155/2014/951478
Effects of stingless bee propolis on experimental asthma
Abstract
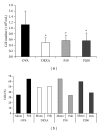
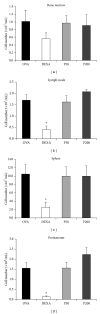
Bee products have been used empirically for centuries, especially for the treatment of respiratory diseases. The present study evaluated the effect of treatment with a propolis hydroalcoholic extract (PHE) produced by Scaptotrigona aff. postica stingless bee in a murine asthma model. BALB/c mice were immunized twice with ovalbumin (OVA) subcutaneously. After 14 days, they were intranasally challenged with OVA. Groups P50 and P200 received PHE by gavage at doses of 50 and 200 mg/kg, respectively. The DEXA group was treated with intraperitoneal injection of dexamethasone. The OVA group received only water. The mice were treated daily for two weeks and then they were immunized a second time with intranasal OVA. The treatment with PHE decreased the cell number in the bronchoalveolar fluid (BAL). Histological analysis showed reduced peribronchovascular inflammation after treatment with PHE especially the infiltration of polymorphonuclear cells. In addition, the concentration of interferon- γ (IFN- γ ) in the serum was decreased. These results were similar to those obtained with dexamethasone. Treatment with S. aff postica propolis reduced the pathology associated with murine asthma due an inhibition of inflammatory cells migration to the alveolar space and the systemic progression of the allergic inflammation.
Figures


References
-
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal. 2008;31(1):143–178. - PubMed
-
- Stirbulov R, Bernd LAG, Solé D. IV Brazilian Guidelines for the Treatment of Asthma (Diretrizes Brasileiras para o Manejo da Asma) Revista Brasileira de Alergia e Imunopatologia. 2006;29(5):222–245.
-
- Bochner BS, Busse WW. Allergy and asthma. The Journal of Allergy and Clinical Immunology. 2005;115(5):953–959. - PubMed
-
- Ten Hacken NH, Oosterhoff Y, Kauffman HF, et al. Elevated serum interferon-γ in atopic asthma correlates with increased airways responsiveness and circadian peak expiratory flow variation. European Respiratory Journal. 1998;11(2):312–316. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

