Highly frequent and enhanced injection site reaction induced by peripheral venous injection of fosaprepitant in anthracycline-treated patients
- PMID: 24799957
- PMCID: PMC4007527
- DOI: 10.7150/jca.7706
Highly frequent and enhanced injection site reaction induced by peripheral venous injection of fosaprepitant in anthracycline-treated patients
Abstract
Background: Fosaprepitant-associated injection site reaction (ISR) has been reported in patients treated with cisplatin, an irritant drug. We conducted this retrospective study to clarify the incidence and symptoms of fosaprepitant-associated ISR in patients treated with anthracycline.
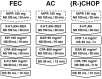
Patients and methods: Fifty six patients receiving 159 injections administering doxorubicin/cyclophosphamide (AC), fluorouracil/epirubicin/cyclophosphamide (FEC), or rituximab/cyclophosphamide/doxorubicin/vincristine/prednisolone (R-)CHOP regimen through a peripheral vein at ambulatory treatment centers reviewed for this study from patients' medical records. Incidence of ISR was compared between 24 patients with fosaprepitant injection (fosaprepitant group) and 32 patients without fosaprepitant (control group). Frequency and symptoms of ISR per injection were also compared between 61 injections with fosaprepitant and 98 injections without fosaprepitant.
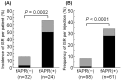
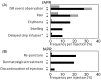
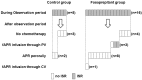
Results: Both the ISR incidence rate per patient and per injection were significantly higher in the fosaprepitant group than in the control group (67% vs. 16%; P=0.0002, 34% vs. 8.2%; P<0.0001, respectively). By multivariate analysis, fosaprepitant injection was found to be a significant independent variable correlated with ISR risk. Symptoms observed in 61 injections of fosaprepitant were pain (n=14, 23%), erythema (n=10, 16%), swelling (n=6, 10%), and delayed drip infusion (n=6, 10%). After the observation period, no ISR occurred when the administration route was changed to central venous injection or oral aprepitant was administered despite the continuation of chemotherapy.
Conclusion: ISR occurred more frequently and severely when fosaprepitant was injected through the peripheral vein in patients treated with anthracyclines compared to those without fosaprepitant.
Keywords: Antiemesis; anthracycline; fosaprepitant; injection site reaction; venous toxicity.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Hesketh PJ, Grunberg SM, Gralla RJ. et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin - the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–9. - PubMed
-
- Poli-Bigelli S, Rodrigues-Pereira J, Carides AD. et al. Addition of the neurokinin-1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy induced nausea and vomiting: results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–8. - PubMed
-
- Warr DG, Hesketh PJ, Gralla RJ. et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–30. - PubMed
-
- Lasseter KC, Gambale J, Jin B. et al. Tolerability of fosaprepitant and bio equivalency to aprepitant in healthy subjects. J Clin Pharmacol. 2007;47:834–40. - PubMed
-
- Grunberg S, Chua D, Maru A. et al. Single-Dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol-EASE. J Clin Oncol. 2011;29:1495–501. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

