Murine model to study brain, behavior and immunity during hepatic encephalopathy
- PMID: 24799993
- PMCID: PMC4009480
- DOI: 10.4254/wjh.v6.i4.243
Murine model to study brain, behavior and immunity during hepatic encephalopathy
Abstract
Aim: To propose an alternative model of hepatic encephalopathy (HE) in mice, resembling the human features of the disease.
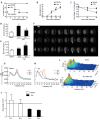
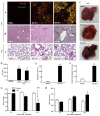
Methods: Mice received two consecutive intraperitoneal injections of thioacetamide (TAA) at low dosage (300 mg/kg). Liver injury was assessed by serum transaminase levels (ALT) and liver histology (hematoxylin and eosin). Neutrophil infiltration was estimated by confocal liver intravital microscopy. Coagulopathy was evaluated using prolonged prothrombin and partial thromboplastin time. Hemodynamic parameters were measured through tail cuff. Ammonia levels were quantified in serum and brain samples. Electroencephalography (EEG) and psychomotor activity score were performed to show brain function. Brain edema was evaluated using magnetic resonance imaging.
Results: Mice submitted to the TAA regime developed massive liver injury, as shown by elevation of serum ALT levels and a high degree of liver necrosis. An intense hepatic neutrophil accumulation occurred in response to TAA-induced liver injury. This led to mice mortality and weight loss, which was associated with severe coagulopathy. Furthermore, TAA-treated mice presented with increased serum and cerebral levels of ammonia, in parallel with alterations in EEG spectrum and discrete brain edema, as shown by magnetic resonance imaging. In agreement with this, neuropsychomotor abnormalities ensued 36 h after TAA, fulfilling several HE features observed in humans. In this context of liver injury and neurological dysfunction, we observed lung inflammation and alterations in blood pressure and heart rate that were indicative of multiple organ dysfunction syndrome.
Conclusion: In summary, we describe a new murine model of hepatic encephalopathy comprising multiple features of the disease in humans, which may provide new insights for treatment.
Keywords: Cerebral herniation; Hepatic encephalopathy; Intracranial hypertension; Liver injury; Neurological dysfunction; Neuropsychomotor abnormalities; Thioacetamide.
Figures
Similar articles
-
Co-administration of C-Phycocyanin ameliorates thioacetamide-induced hepatic encephalopathy in Wistar rats.J Neurol Sci. 2007 Jan 15;252(1):67-75. doi: 10.1016/j.jns.2006.10.014. Epub 2006 Dec 12. J Neurol Sci. 2007. PMID: 17169376
-
Ameliorative effects of rutin on hepatic encephalopathy-induced by thioacetamide or gamma irradiation.J Photochem Photobiol B. 2017 Jul;172:20-27. doi: 10.1016/j.jphotobiol.2017.05.005. Epub 2017 May 8. J Photochem Photobiol B. 2017. PMID: 28505498
-
[Expression of aquaporin-4 during brain edema in rats with thioacetamide-induced acute encephalopathy].Zhonghua Yi Xue Za Zhi. 2011 Sep 27;91(36):2573-7. Zhonghua Yi Xue Za Zhi. 2011. PMID: 22321890 Chinese.
-
Simvastatin for rats with thioacetamide-induced liver failure and encephalopathy.J Gastroenterol Hepatol. 2008 Jul;23(7 Pt 2):e236-42. doi: 10.1111/j.1440-1746.2007.04988.x. Epub 2007 Jun 15. J Gastroenterol Hepatol. 2008. PMID: 17573832
-
BabaoDan cures hepatic encephalopathy by decreasing ammonia levels and alleviating inflammation in rats.J Ethnopharmacol. 2020 Mar 1;249:112301. doi: 10.1016/j.jep.2019.112301. Epub 2019 Oct 14. J Ethnopharmacol. 2020. PMID: 31622746
Cited by
-
The Influence of Finasteride on Mean and Relative Spectral Density of EEG Bands in Rat Model of Thioacetamide-Induced Hepatic Encephalopathy.Neurotox Res. 2016 Aug;30(2):150-8. doi: 10.1007/s12640-016-9610-z. Epub 2016 Mar 7. Neurotox Res. 2016. PMID: 26951455
-
Sepsis Encephalopathy Is Partly Mediated by miR370-3p-Induced Mitochondrial Injury but Attenuated by BAM15 in Cecal Ligation and Puncture Sepsis Male Mice.Int J Mol Sci. 2022 May 13;23(10):5445. doi: 10.3390/ijms23105445. Int J Mol Sci. 2022. PMID: 35628259 Free PMC article.
-
Cannabidiol rescues acute hepatic toxicity and seizure induced by cocaine.Mediators Inflamm. 2015;2015:523418. doi: 10.1155/2015/523418. Epub 2015 Apr 27. Mediators Inflamm. 2015. PMID: 25999668 Free PMC article.
-
The New Phytocomplex AL0042 Extracted from Red Orange By-Products Inhibits the Minimal Hepatic Encephalopathy in Mice Induced by Thioacetamide.Biomedicines. 2025 Mar 11;13(3):686. doi: 10.3390/biomedicines13030686. Biomedicines. 2025. PMID: 40149662 Free PMC article.
-
Drug-induced-acute liver failure: A critical appraisal of the thioacetamide model for the study of hepatic encephalopathy.Toxicol Rep. 2021 Apr 30;8:962-970. doi: 10.1016/j.toxrep.2021.04.011. eCollection 2021. Toxicol Rep. 2021. PMID: 34026559 Free PMC article. Review.
References
-
- Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. - PubMed
-
- Ichai P, Samuel D. Epidemiology of liver failure. Clin Res Hepatol Gastroenterol. 2011;35:610–617. - PubMed
-
- Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, Rela M, Heaton N, O’Grady JG, Wendon J, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. - PubMed
-
- Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36–45. - PubMed
-
- Vaquero J, Chung C, Blei AT. Brain edema in acute liver failure. A window to the pathogenesis of hepatic encephalopathy. Ann Hepatol. 2003;2:12–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

