Stem cells in the nervous system
- PMID: 24800720
- PMCID: PMC4197112
- DOI: 10.1097/PHM.0000000000000111
Stem cells in the nervous system
Abstract
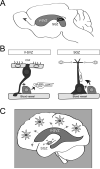
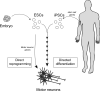
Given their capacity to regenerate cells lost through injury or disease, stem cells offer new vistas into possible treatments for degenerative diseases and their underlying causes. As such, stem cell biology is emerging as a driving force behind many studies in regenerative medicine. This review focuses on the current understanding of the applications of stem cells in treating ailments of the human brain, with an emphasis on neurodegenerative diseases. Two types of neural stem cells are discussed: endogenous neural stem cells residing within the adult brain and pluripotent stem cells capable of forming neural cells in culture. Endogenous neural stem cells give rise to neurons throughout life, but they are restricted to specialized regions in the brain. Elucidating the molecular mechanisms regulating these cells is key in determining their therapeutic potential as well as finding mechanisms to activate dormant stem cells outside these specialized microdomains. In parallel, patient-derived stem cells can be used to generate neural cells in culture, providing new tools for disease modeling, drug testing, and cell-based therapies. Turning these technologies into viable treatments will require the integration of basic science with clinical skills in rehabilitation.
Figures



References
-
- Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3(5):480–483. doi:10.1016/j.stem.2008.10.007. - PubMed
-
- Masui K, Suzuki SO, Torisu R, Goldman JE, Canoll P, Iwaki T. Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. Glia. 2010;58(9):1050–1065. doi:10.1002/glia.20986. - PubMed
-
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6(6):425–436. doi:10.1038/nrc1889. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

