Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections
- PMID: 24800825
- PMCID: PMC7091848
- DOI: 10.1038/ncomms4594
Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections
Abstract
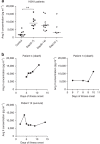
The potential for avian influenza H5N1 outbreaks has increased in recent years. Thus, it is paramount to develop novel strategies to alleviate death rates. Here we show that avian influenza A H5N1-infected patients exhibit markedly increased serum levels of angiotensin II. High serum levels of angiotensin II appear to be linked to the severity and lethality of infection, at least in some patients. In experimental mouse models, infection with highly pathogenic avian influenza A H5N1 virus results in downregulation of angiotensin-converting enzyme 2 (ACE2) expression in the lung and increased serum angiotensin II levels. Genetic inactivation of ACE2 causes severe lung injury in H5N1-challenged mice, confirming a role of ACE2 in H5N1-induced lung pathologies. Administration of recombinant human ACE2 ameliorates avian influenza H5N1 virus-induced lung injury in mice. Our data link H5N1 virus-induced acute lung failure to ACE2 and provide a potential treatment strategy to address future flu pandemics.
Conflict of interest statement
J.M.P. holds shares in Apeiron Biologics which together with GSK are developing recombinant human ACE2 for acute lung injury patients. The remaining authors have no competing financial interests to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

