Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients
- PMID: 24800963
- PMCID: PMC7091598
- DOI: 10.1038/ncomms4595
Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients
Abstract
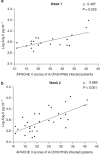
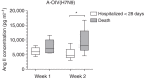
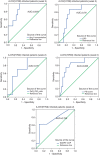
A novel influenza A (H7N9) virus of avian origin emerged in eastern China in the spring of 2013. This virus causes severe disease in humans, including acute and often lethal respiratory failure. As of January 2014, 275 cases of H7N9-infected patients had been reported, highlighting the urgency of identifying biomarkers for predicting disease severity and fatal outcomes. Here, we show that plasma levels of angiotensin II, a major regulatory peptide of the renin-angiotensin system, are markedly elevated in H7N9 patients and are associated with disease progression. Moreover, the sustained high levels of angiotensin II in these patients are strongly correlated with mortality. The predictive value of angiotensin II is higher than that of C-reactive protein and some clinical parameters such as the PaO2/FiO2 ratio (partial pressure of arterial oxygen to the fraction of inspired oxygen). Our findings indicate that angiotensin II is a biomarker for lethality in flu infections.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
The Serum Profile of Hypercytokinemia Factors Identified in H7N9-Infected Patients can Predict Fatal Outcomes.Sci Rep. 2015 Jun 1;5:10942. doi: 10.1038/srep10942. Sci Rep. 2015. PMID: 26028236 Free PMC article.
-
Comparison between human infections caused by highly and low pathogenic H7N9 avian influenza viruses in Wave Five: Clinical and virological findings.J Infect. 2019 Mar;78(3):241-248. doi: 10.1016/j.jinf.2019.01.005. Epub 2019 Jan 18. J Infect. 2019. PMID: 30664912
-
Host immunological response and factors associated with clinical outcome in patients with the novel influenza A H7N9 infection.Clin Microbiol Infect. 2014 Aug;20(8):O493-500. doi: 10.1111/1469-0691.12505. Epub 2014 Jan 21. Clin Microbiol Infect. 2014. PMID: 24350809
-
H7N9: preparing for the unexpected in influenza.Annu Rev Med. 2015;66:361-71. doi: 10.1146/annurev-med-010714-112311. Epub 2014 Oct 29. Annu Rev Med. 2015. PMID: 25386931 Review.
-
The Effects of Genetic Variation on H7N9 Avian Influenza Virus Pathogenicity.Viruses. 2020 Oct 28;12(11):1220. doi: 10.3390/v12111220. Viruses. 2020. PMID: 33126529 Free PMC article. Review.
Cited by
-
The Impact of the Renin-Angiotensin-Aldosterone System on Inflammation, Coagulation, and Atherothrombotic Complications, and to Aggravated COVID-19.Front Pharmacol. 2021 Jun 17;12:640185. doi: 10.3389/fphar.2021.640185. eCollection 2021. Front Pharmacol. 2021. PMID: 34220496 Free PMC article. Review.
-
Respiratory infectious phenotypes in acute exacerbation of COPD: an aid to length of stay and COPD Assessment Test.Int J Chron Obstruct Pulmon Dis. 2015 Oct 20;10:2257-63. doi: 10.2147/COPD.S92160. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26527871 Free PMC article.
-
Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors in COVID-19: Meta-analysis/Meta-regression Adjusted for Confounding Factors.CJC Open. 2021 Jul;3(7):965-975. doi: 10.1016/j.cjco.2021.03.001. Epub 2021 Apr 6. CJC Open. 2021. PMID: 33842874 Free PMC article. Review.
-
Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation.Aging Cell. 2020 Jul;19(7):e13168. doi: 10.1111/acel.13168. Epub 2020 Jun 19. Aging Cell. 2020. PMID: 32558150 Free PMC article.
-
Viral Infection and Cardiovascular Disease: Implications for the Molecular Basis of COVID-19 Pathogenesis.Int J Mol Sci. 2021 Feb 7;22(4):1659. doi: 10.3390/ijms22041659. Int J Mol Sci. 2021. PMID: 33562193 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

