Modulation of neural circuits: how stimulus context shapes innate behavior in Drosophila
- PMID: 24801064
- PMCID: PMC4219929
- DOI: 10.1016/j.conb.2014.04.008
Modulation of neural circuits: how stimulus context shapes innate behavior in Drosophila
Abstract
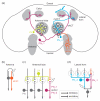
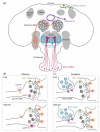
Remarkable advances have been made in recent years in our understanding of innate behavior and the underlying neural circuits. In particular, a wealth of neuromodulatory mechanisms have been uncovered that can alter the input-output relationship of a hereditary neural circuit. It is now clear that this inbuilt flexibility allows animals to modify their behavioral responses according to environmental cues, metabolic demands and physiological states. Here, we discuss recent insights into how modulation of neural circuits impacts innate behavior, with a special focus on how environmental cues and internal physiological states shape different aspects of feeding behavior in Drosophila.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–65. - PubMed
-
- Riffell JA. Olfactory ecology and the processing of complex mixtures. Curr. Opin. Neurobiol. 2012;22:236–42. - PubMed
-
- Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat. Rev. Genet. 2002;3:589–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

