Rhodopsin in the rod surface membrane regenerates more rapidly than bulk rhodopsin in the disc membranes in vivo
- PMID: 24801306
- PMCID: PMC4221820
- DOI: 10.1113/jphysiol.2014.272518
Rhodopsin in the rod surface membrane regenerates more rapidly than bulk rhodopsin in the disc membranes in vivo
Abstract
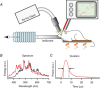
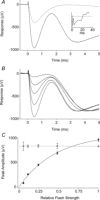
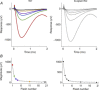
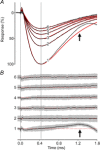
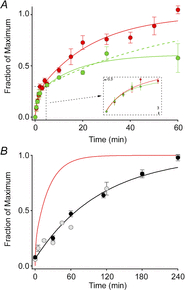
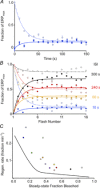
Sustained vertebrate vision requires that opsin chromophores isomerized by light to the all-trans form be replaced with 11-cis retinal to regenerate the visual pigment. We have characterized the early receptor potential (ERP), a component of the electroretinogram arising from photoisomerization-induced charge displacements in plasma membrane visual pigment, and used it to measure pigment bleaching and regeneration in living mice. The mouse ERP was characterized by an outward 'R2' charge displacement with a time constant of 215 μs that discharged through a membrane with an apparent time constant of ∼0.6 ms. After complete bleaching of rhodopsin, the ERP recovered in two phases. The initial, faster phase had a time constant of ∼1 min, accounted for ∼20% of the total, and was not dependent on the level of expression of the retinal pigment epithelium isomerase, Rpe65. The slower, complementary phase had a time constant of 23 min in wild-type (WT) mice (C57Bl/6) and was substantially slowed in Rpe65(+/-) mice. Comparison of the ERPs of a mouse line expressing 150% of the normal level of cone M-opsin with those of WT mice revealed that M-opsin contributed 26% of the total WT ERP in these experiments, with the remaining 74% arising from rhodopsin. Thus, the fast regenerating fraction (20%) corresponds approximately to the fraction of the total ERP independently estimated to arise from M-opsin. Because both phases of the ERP recover substantially faster than previous measurements of bulk rhodopsin regeneration in living mice, we conclude that delivery of the highly hydrophobic 11-cis retinal to the interior of rod photoreceptors appears to be retarded by transit across the cytoplasmic gap between plasma and disc membranes.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures
Similar articles
-
Protection of cone photoreceptor M-opsin degradation with 9-cis-β-carotene-rich alga Dunaliella bardawil in Rpe65(-/-) mouse retinal explant culture.Curr Eye Res. 2014 Dec;39(12):1221-31. doi: 10.3109/02713683.2014.907430. Epub 2014 Jul 9. Curr Eye Res. 2014. PMID: 25006880
-
Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice.J Physiol. 2005 Oct 1;568(Pt 1):83-95. doi: 10.1113/jphysiol.2005.091942. Epub 2005 Jul 1. J Physiol. 2005. PMID: 15994181 Free PMC article.
-
Correlation of regenerable opsin with rod ERG signal in Rpe65-/- mice during development and aging.Invest Ophthalmol Vis Sci. 2003 Jan;44(1):310-5. doi: 10.1167/iovs.02-0567. Invest Ophthalmol Vis Sci. 2003. PMID: 12506090
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
-
Retinal photoisomerase: role in invertebrate visual cells.J Photochem Photobiol B. 1992 Apr 15;13(1):5-17. doi: 10.1016/1011-1344(92)80035-t. J Photochem Photobiol B. 1992. PMID: 1403367 Review.
Cited by
-
Retinoids and Retinal Diseases.Annu Rev Vis Sci. 2016 Oct;2:197-234. doi: 10.1146/annurev-vision-111815-114407. Epub 2016 Jul 18. Annu Rev Vis Sci. 2016. PMID: 27917399 Free PMC article. Review.
-
Rhodopsin kinase and arrestin binding control the decay of photoactivated rhodopsin and dark adaptation of mouse rods.J Gen Physiol. 2016 Jul;148(1):1-11. doi: 10.1085/jgp.201511538. J Gen Physiol. 2016. PMID: 27353443 Free PMC article.
-
Optoretinography reveals rapid rod photoreceptor movement upon photoisomerization.bioRxiv [Preprint]. 2025 Mar 25:2025.03.22.644466. doi: 10.1101/2025.03.22.644466. bioRxiv. 2025. PMID: 40196674 Free PMC article. Preprint.
-
Nano-scale resolution of native retinal rod disk membranes reveals differences in lipid composition.J Cell Biol. 2021 Aug 2;220(8):e202101063. doi: 10.1083/jcb.202101063. Epub 2021 Jun 16. J Cell Biol. 2021. PMID: 34132745 Free PMC article.
-
Role of ω3 polyunsaturated fatty acids in diabetic retinopathy: a morphological and metabolically cross talk among blood retina barriers damage, autoimmunity and chronic inflammation.Lipids Health Dis. 2019 May 15;18(1):114. doi: 10.1186/s12944-019-1049-9. Lipids Health Dis. 2019. PMID: 31092270 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

