Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils
- PMID: 24801612
- PMCID: PMC4119597
- DOI: 10.1016/j.biocel.2014.04.020
Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils
Abstract
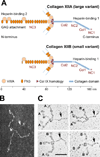
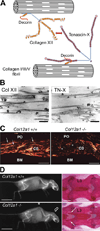
Collagen XII, largest member of the fibril-associated collagens with interrupted triple helix (FACIT) family, assembles from three identical α-chains encoded by the COL12A1 gene. The molecule consists of three threadlike N-terminal noncollagenous NC3 domains, joined by disulfide bonds and a short interrupted collagen triple helix toward the C-terminus. Splice variants differ considerably in size and properties: "small" collagen XIIB (220 kDa subunit) is similar to collagen XIV, whereas collagen XIIA (350 kDa) has a much larger NC3 domain carrying glycosaminoglycan chains. Collagen XII binds to collagen I-containing fibrils via its collagenous domain, whereas its large noncollagenous arms interact with other matrix proteins such as tenascin-X. In dense connective tissues and bone, collagen XII is thought to regulate organization and mechanical properties of collagen fibril bundles. Accordingly, recent findings show that collagen XII mutations cause Ehlers-Danlos/myopathy overlap syndrome associated with skeletal abnormalities and muscle weakness in mice and humans.
Keywords: Bethlem myopathy; Collagen XII; Collagen fiber; Ehlers–Danlos syndrome; Osteogenesis.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function.Matrix Biol. 2021 Jan;95:52-67. doi: 10.1016/j.matbio.2020.10.004. Epub 2020 Oct 20. Matrix Biol. 2021. PMID: 33096204 Free PMC article.
-
Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers-Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix.Genet Med. 2020 Jan;22(1):112-123. doi: 10.1038/s41436-019-0599-6. Epub 2019 Jul 5. Genet Med. 2020. PMID: 31273343
-
Collagen XII-Related Myopathy: An Emerging Spectrum of Extracellular Matrix-Related Myopathy.Neurol India. 2023 Nov-Dec;71(6):1257-1259. doi: 10.4103/0028-3886.391402. Neurol India. 2023. PMID: 38174471
-
Collagen XII mediated cellular and extracellular mechanisms in development, regeneration, and disease.Front Cell Dev Biol. 2023 Mar 2;11:1129000. doi: 10.3389/fcell.2023.1129000. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36936682 Free PMC article. Review.
-
[A current viewpoint on structure and evolution of collagens. II. The fibril-associated collagens with interrupted triple helices].Zh Evol Biokhim Fiziol. 2014 Jul-Aug;50(4):245-54. Zh Evol Biokhim Fiziol. 2014. PMID: 25775860 Review. Russian.
Cited by
-
Composition, structure and function of the corneal stroma.Exp Eye Res. 2020 Sep;198:108137. doi: 10.1016/j.exer.2020.108137. Epub 2020 Jul 11. Exp Eye Res. 2020. PMID: 32663498 Free PMC article. Review.
-
The Transcriptomics of the Human Vein Transformation After Arteriovenous Fistula Anastomosis Uncovers Layer-Specific Remodeling and Hallmarks of Maturation Failure.Kidney Int Rep. 2023 Jan 17;8(4):837-850. doi: 10.1016/j.ekir.2023.01.008. eCollection 2023 Apr. Kidney Int Rep. 2023. PMID: 37069981 Free PMC article.
-
Adipose matrix complex: a high-rigidity collagen-rich adipose-derived material for fat grafting.Aging (Albany NY). 2021 Jun 9;13(11):14910-14923. doi: 10.18632/aging.203120. Epub 2021 Jun 9. Aging (Albany NY). 2021. PMID: 34111029 Free PMC article.
-
Unusual Suspects: Bone and Cartilage ECM Proteins as Carcinoma Facilitators.Cancers (Basel). 2023 Jan 27;15(3):791. doi: 10.3390/cancers15030791. Cancers (Basel). 2023. PMID: 36765749 Free PMC article. Review.
-
Multifaceted Interweaving Between Extracellular Matrix, Insulin Resistance, and Skeletal Muscle.Cells. 2018 Sep 22;7(10):148. doi: 10.3390/cells7100148. Cells. 2018. PMID: 30249008 Free PMC article. Review.
References
-
- Anderson S, SundarRaj S, Fite D, Wessel H, SundarRaj N. Developmentally regulated appearance of spliced variants of type XII collagen in the cornea. Invest Ophthalmol Vis Sci. 2000;41:55–63. - PubMed
-
- Arai K, Nagashima Y, Takemoto T, Nishiyama T. Mechanical strain increases expression of type XII collagen in murine osteoblastic MC3T3-E1 cells. Cell Struct Funct. 2008;33:203–210. - PubMed
-
- Berthod F, Germain L, Guignard R, Lethias C, Garrone R, Damour O, van der Rest M, Auger FA. Differential expression of collagens XII and XIV in human skin and in reconstructed skin. J Invest Dermatol. 1997;108:737–742. - PubMed
-
- Chiquet M, Mumenthaler U, Wittwer M, Jin W, Koch M. The chick and human collagen alpha1(XII) gene promoter--activity of highly conserved regions around the first exon and in the first intron. Eur J Biochem. 1998;257:362–371. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

