A synaptic signature for ON- and OFF-center parasol ganglion cells of the primate retina
- PMID: 24801624
- PMCID: PMC4503220
- DOI: 10.1017/S0952523813000461
A synaptic signature for ON- and OFF-center parasol ganglion cells of the primate retina
Abstract
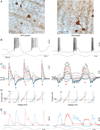
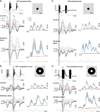
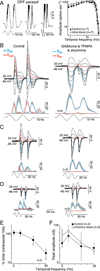
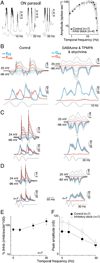
In the primate retina, parasol ganglion cells contribute to the primary visual pathway via the magnocellular division of the lateral geniculate nucleus, display ON and OFF concentric receptive field structure, nonlinear spatial summation, and high achromatic temporal-contrast sensitivity. Parasol cells may be homologous to the alpha-Y cells of nonprimate mammals where evidence suggests that N-methyl-D-aspartate (NMDA) receptor-mediated synaptic excitation as well as glycinergic disinhibition play critical roles in contrast sensitivity, acting asymmetrically in OFF- but not ON-pathways. Here, light-evoked synaptic currents were recorded in the macaque monkey retina in vitro to examine the circuitry underlying parasol cell receptive field properties. Synaptic excitation in both ON and OFF types was mediated by NMDA as well as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate glutamate receptors. The NMDA-mediated current-voltage relationship suggested high Mg2+ affinity such that at physiological potentials, NMDA receptors contributed ∼20% of the total excitatory conductance evoked by moderate stimulus contrasts and temporal frequencies. Postsynaptic inhibition in both ON and OFF cells was dominated by a large glycinergic "crossover" conductance, with a relatively small contribution from GABAergic feedforward inhibition. However, crossover inhibition was largely rectified, greatly diminished at low stimulus contrasts, and did not contribute, via disinhibition, to contrast sensitivity. In addition, attenuation of GABAergic and glycinergic synaptic inhibition left center-surround and Y-type receptive field structure and high temporal sensitivity fundamentally intact and clearly derived from modulation of excitatory bipolar cell output. Thus, the characteristic spatial and temporal-contrast sensitivity of the primate parasol cell arises presynaptically and is governed primarily by modulation of the large AMPA/kainate receptor-mediated excitatory conductance. Moreover, the negative feedback responsible for the receptive field surround must derive from a nonGABAergic mechanism.
Figures







References
-
- Abbott CJ, Percival KA, Martin PR, Grunert U. Amacrine and bipolar inputs to midget and parasol ganglion cells in marmoset retina. Visual Neuroscience. 2012;29:157–168. - PubMed
-
- Borg-Graham LJ. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nature Nueroscience. 2001;4:176–183. - PubMed
-
- Boos R, Muller F, Wassle H. Actions of excitatory amino acids on brisk ganglion cells in the cat retina. Journal of Neurophysiology. 1990;64:1368–1379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

