Pathway of programmed cell death and oxidative stress induced by β-hydroxybutyrate in dairy cow abomasum smooth muscle cells and in mouse gastric smooth muscle
- PMID: 24801711
- PMCID: PMC4011855
- DOI: 10.1371/journal.pone.0096775
Pathway of programmed cell death and oxidative stress induced by β-hydroxybutyrate in dairy cow abomasum smooth muscle cells and in mouse gastric smooth muscle
Abstract
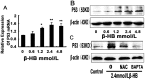
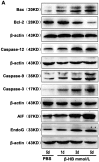
The administration of exogenous β-hydroxybutyrate (β-HB), as well as fasting and caloric restriction, is a condition associated with β-HB abundance and decreased appetite in animals. Increased β-HB and decreased appetite exist simultaneously in some diseases, such as bovine left displaced abomasums (LDA) and human chronic gastritis. However, the effects of β-HB on stomach injuries have not been explored. To elucidate the possible effects of exogenous β-HB on the stomach, mice were injected intraperitoneally with β-HB, and bovine abomasum smooth muscle cells (BSMCs) were treated with different concentrations of β-HB. We found that β-HB induced BSMCs endoplasmic reticulum- and mitochondria-mediated apoptotic cell death. β-HB promoted Bax expression and caspase-12, -9, and -3 activation while blocking Bcl-2 expression. β-HB also promoted AIF, EndoG release and p53 expression. β-HB acted on key molecules in the apoptotic cell death pathway and increased p38 and c-June NH2-terminal kinase phosphorylation while inhibiting ERK phosphorylation and PCNA expression. β-HB upregulated P27 and P21 mRNA levels while downregulating cyclin and CDK mRNA levels, arresting the cell cycle. These results suggest that BSMCs treated with β-HB can induce oxidative stress, which can be prevented by intracellular calcium chelators BAPTA/AM but not antioxidant NAC. Additionally, these results suggest that β-HB causes ROS generation through a Ca2+-dependent mechanism and that intracellular Ca2+ levels play a critical role in β-HB -induced apoptotic cell death. The impact of β-HB on programmed cell death and oxidative stress in vivo was confirmed in murine experiments. For the first time, we show oxidative stress effects of β-HB on smooth muscle. We propose that β-HB is a possible cause of some stomach diseases, including bovine LDA.
Conflict of interest statement
Figures







Similar articles
-
Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress.Biomaterials. 2011 Aug;32(23):5438-58. doi: 10.1016/j.biomaterials.2011.04.024. Epub 2011 May 2. Biomaterials. 2011. PMID: 21543116
-
The roles of endoplasmic reticulum stress and Ca2+ on rhein-induced apoptosis in A-549 human lung cancer cells.Anticancer Res. 2009 Jan;29(1):309-18. Anticancer Res. 2009. PMID: 19331167
-
Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation.PLoS One. 2013;8(2):e54374. doi: 10.1371/journal.pone.0054374. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405080 Free PMC article.
-
Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species.Circ Res. 2009 Dec 4;105(12):1204-12. doi: 10.1161/CIRCRESAHA.109.204172. Epub 2009 Oct 22. Circ Res. 2009. PMID: 19850941
-
Dual effect of thapsigargin on cell death in porcine aortic smooth muscle cells.Am J Physiol Cell Physiol. 2007 Jan;292(1):C383-95. doi: 10.1152/ajpcell.00069.2006. Am J Physiol Cell Physiol. 2007. PMID: 17218371
Cited by
-
β-hydroxybutyrate impairs bovine oocyte maturation via pyruvate dehydrogenase (PDH) associated energy metabolism abnormality.Front Pharmacol. 2023 Aug 11;14:1243243. doi: 10.3389/fphar.2023.1243243. eCollection 2023. Front Pharmacol. 2023. PMID: 37637420 Free PMC article.
-
Effect of Berberine from Coptis chinensis on Apoptosis of Intestinal Epithelial Cells in a Mouse Model of Ulcerative Colitis: Role of Endoplasmic Reticulum Stress.Evid Based Complement Alternat Med. 2020 Apr 24;2020:3784671. doi: 10.1155/2020/3784671. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32382284 Free PMC article.
-
Sirtuin 3 regulation: a target to alleviate β-hydroxybutyric acid-induced mitochondrial dysfunction in bovine granulosa cells.J Anim Sci Biotechnol. 2023 Feb 14;14(1):18. doi: 10.1186/s40104-022-00825-w. J Anim Sci Biotechnol. 2023. PMID: 36788581 Free PMC article.
-
The Dynamic Transcription Profiles of Proliferating Bovine Ovarian Granulosa When Exposed to Increased Levels of β-Hydroxybutyric Acid.Front Vet Sci. 2022 Aug 5;9:915956. doi: 10.3389/fvets.2022.915956. eCollection 2022. Front Vet Sci. 2022. PMID: 35990259 Free PMC article.
-
Persistently high concentrations of β-hydroxybutyrate affect hepatic SOD2 expression and blood SOD activity in high-yielding dairy cows.BMC Vet Res. 2025 Jan 9;21(1):12. doi: 10.1186/s12917-024-04464-3. BMC Vet Res. 2025. PMID: 39789575 Free PMC article.
References
-
- Byard RW, Zhou C (2010) Erosive gastritis, Armanni-Ebstein phenomenon and diabetic ketoacidosis. Forensic Sci Med Pathol 6: 304–306. - PubMed
-
- Laeger T, Metges CC, Kuhla B (2010) Role of beta-hydroxybutyric acid in the central regulation of energy balance. Appetite 54: 450–455. - PubMed
-
- LeBlanc SJ, Leslie KE, Duffield TF (2005) Metabolic predictors of displaced abomasum in dairy cattle. J Dairy Sci 88: 159–170. - PubMed
-
- Rehage J, Mertens M, Stockhofe-Zurwieden N, Kaske M, Scholz H (1996) Post surgical convalescence of dairy cows with left abomasal displacement in relation to fatty liver. Schweiz Arch Tierheilkd 138: 361–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

