Haploinsufficiency of Def activates p53-dependent TGFβ signalling and causes scar formation after partial hepatectomy
- PMID: 24801718
- PMCID: PMC4011785
- DOI: 10.1371/journal.pone.0096576
Haploinsufficiency of Def activates p53-dependent TGFβ signalling and causes scar formation after partial hepatectomy
Abstract
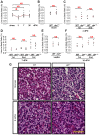
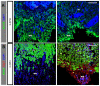
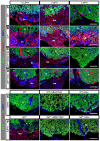
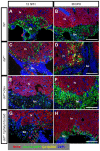
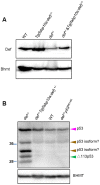
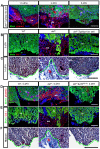
The metazoan liver exhibits a remarkable capacity to regenerate lost liver mass without leaving a scar following partial hepatectomy (PH). Whilst previous studies have identified components of several different signaling pathways that are essential for activation of hepatocyte proliferation during liver regeneration, the mechanisms that enable such regeneration to occur without accompanying scar formation remain poorly understood. Here we use the adult zebrafish liver, which can regenerate within two weeks following PH, as a new genetic model to address this important question. We focus on the role of Digestive-organ-expansion-factor (Def), a nucleolar protein which has recently been shown to complex with calpain3 (Capn3) to mediate p53 degradation specifically in the nucleolus, in liver regeneration. Firstly, we show that Def expression is up-regulated in the wild-type liver following amputation, and that the defhi429/+ heteroozygous mutant (def+/-) suffers from haploinsufficiency of Def in the liver. We then show that the expression of pro-inflammatory cytokines is up-regulated in the def+/- liver, which leads to distortion of the migration and the clearance of leukocytes after PH. Transforming growth factor β (TGFβ) signalling is thus activated in the wound epidermis in def+/- due to a prolonged inflammatory response, which leads to fibrosis at the amputation site. Fibrotic scar formation in def+/- is blocked by the over-expression of Def, by the loss-of-function of p53, and by treatment with anti-inflammation drug dexamethasone or TGFβ-signalling inhibitor SB431542. We finally show that the Def- p53 pathway suppresses fibrotic scar formation, at least in part, through the regulation of the expression of the pro-inflammatory factor, high-mobility group box 1. We conclude that the novel Def- p53 nucleolar pathway functions specifically to prevent a scar formation at the amputation site in a normal amputated liver.
Conflict of interest statement
Figures





Similar articles
-
Def defines a conserved nucleolar pathway that leads p53 to proteasome-independent degradation.Cell Res. 2013 May;23(5):620-34. doi: 10.1038/cr.2013.16. Epub 2013 Jan 29. Cell Res. 2013. PMID: 23357851 Free PMC article.
-
Ribosome biogenesis gene DEF/UTP25 is essential for liver homeostasis and regeneration.Sci China Life Sci. 2020 Nov;63(11):1651-1664. doi: 10.1007/s11427-019-1635-2. Epub 2020 Apr 14. Sci China Life Sci. 2020. PMID: 32303961
-
Capn3 depletion causes Chk1 and Wee1 accumulation and disrupts synchronization of cell cycle reentry during liver regeneration after partial hepatectomy.Cell Regen. 2020 Jun 11;9(1):8. doi: 10.1186/s13619-020-00049-1. Cell Regen. 2020. PMID: 32588143 Free PMC article.
-
Role of p53 family in birth defects: lessons from zebrafish.Birth Defects Res C Embryo Today. 2008 Sep;84(3):215-27. doi: 10.1002/bdrc.20129. Birth Defects Res C Embryo Today. 2008. PMID: 18773461 Review.
-
Liver regeneration biology: Implications for liver tumour therapies.World J Clin Oncol. 2021 Dec 24;12(12):1101-1156. doi: 10.5306/wjco.v12.i12.1101. World J Clin Oncol. 2021. PMID: 35070734 Free PMC article. Review.
Cited by
-
The pre-rRNA processing factor DEF is rate limiting for the pathogenesis of MYCN-driven neuroblastoma.Oncogene. 2017 Jul 6;36(27):3852-3867. doi: 10.1038/onc.2016.527. Epub 2017 Mar 6. Oncogene. 2017. PMID: 28263972 Free PMC article.
-
Sex hormone receptors, calcium-binding protein and Yap1 signaling regulate sex-dependent liver cell proliferation following partial hepatectomy.Dis Model Mech. 2024 Oct 1;17(10):dmm050900. doi: 10.1242/dmm.050900. Epub 2024 Oct 30. Dis Model Mech. 2024. PMID: 39397390 Free PMC article.
-
PPARα activation promotes liver progenitor cell-mediated liver regeneration by suppressing YAP signaling in zebrafish.Sci Rep. 2023 Oct 25;13(1):18312. doi: 10.1038/s41598-023-44935-5. Sci Rep. 2023. PMID: 37880271 Free PMC article.
-
Partial Hepatectomy in Adult Zebrafish.J Vis Exp. 2021 Apr 4;(170):10.3791/62349. doi: 10.3791/62349. J Vis Exp. 2021. PMID: 33871461 Free PMC article.
-
Macrophages in Zebrafish Models of Liver Diseases.Front Immunol. 2019 Dec 4;10:2840. doi: 10.3389/fimmu.2019.02840. eCollection 2019. Front Immunol. 2019. PMID: 31867007 Free PMC article. Review.
References
-
- Taub R (2004) Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5: 836–847. - PubMed
-
- Fausto N, Campbell JS, Riehle KJ (2006) Liver regeneration. Hepatology 43: S45–S53. - PubMed
-
- Costa RH, Kalinichenko VV, Holterman AX, Wang X (2003) Transcription factors in liver development, differentiation, and regeneration. Hepatology 38: 1331–1347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

