Efficacy and safety of olanzapine/fluoxetine combination vs fluoxetine monotherapy following successful combination therapy of treatment-resistant major depressive disorder
- PMID: 24801768
- PMCID: PMC4207330
- DOI: 10.1038/npp.2014.101
Efficacy and safety of olanzapine/fluoxetine combination vs fluoxetine monotherapy following successful combination therapy of treatment-resistant major depressive disorder
Abstract
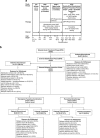
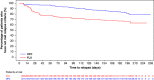
This study assessed prevention of relapse in patients with treatment-resistant depression (TRD) taking olanzapine/fluoxetine combination (OFC). Patients with major depressive disorder (MDD) who failed to satisfactorily respond to ≥ 2 different antidepressants for ≥ 6 weeks within the current MDD episode were acutely treated for 6-8 weeks, followed by stabilization (12 weeks) on OFC. Those who remained stable were randomized to OFC or fluoxetine for up to 27 weeks. Time-to-relapse was the primary efficacy outcome defined as 50% increase in Montgomery-Åsberg Depression Rating Scale score with Clinical Global Impressions-Severity of Depression score of ≥ 4; hospitalization for depression or suicidality; or discontinuation for lack of efficacy or worsening of depression or suicidality. A total of 444 patients were randomized 1:1 to OFC (N=221) or fluoxetine (N=223). Time-to-relapse was significantly longer in OFC-treated patients compared with fluoxetine-treated patients (p<0.001). Treatment-emergent weight gain and some mean and categorical fasting metabolic changes were significantly greater in OFC-treated patients. Clinically significant weight gain (≥ 7%) was observed in 55.7% of patients who remained on OFC throughout the study, including the relapse-prevention phase (up to 47 weeks). There were no significant differences between patients treated with OFC and fluoxetine in extrapyramidal symptoms or serious adverse events. We believe this is the first controlled relapse-prevention study in subjects with TRD that supports continued use of a second-generation antipsychotic beyond stabilization. A thorough assessment of benefits and risks (in particular metabolic changes) associated with continuing treatment with OFC or fluoxetine must be done based on individual patient needs.
Figures
Similar articles
-
An integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depression.J Clin Psychiatry. 2009 Mar;70(3):387-96. doi: 10.4088/jcp.08m04064. Epub 2009 Mar 10. J Clin Psychiatry. 2009. PMID: 19284928
-
A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression.Depress Anxiety. 2006;23(6):364-72. doi: 10.1002/da.20130. Depress Anxiety. 2006. PMID: 16710853 Clinical Trial.
-
Olanzapine/fluoxetine combination for the treatment of mixed depression in bipolar I disorder: a post hoc analysis.J Clin Psychiatry. 2009 Oct;70(10):1424-31. doi: 10.4088/JCP.08m04772gre. J Clin Psychiatry. 2009. PMID: 19906346
-
Efficacy, safety and tolerability of Symbyax for acute-phase management of treatment-resistant depression.Expert Rev Neurother. 2010 May;10(5):651-70. doi: 10.1586/ern.10.44. Expert Rev Neurother. 2010. PMID: 20420487 Review.
-
Drug safety evaluation of olanzapine/fluoxetine combination.Expert Opin Drug Saf. 2014 Aug;13(8):1133-41. doi: 10.1517/14740338.2014.933804. Epub 2014 Jun 27. Expert Opin Drug Saf. 2014. PMID: 24972823 Review.
Cited by
-
A preliminary study of resting brain metabolism in treatment-resistant depression before and after treatment with olanzapine-fluoxetine combination.PLoS One. 2020 Jan 13;15(1):e0226486. doi: 10.1371/journal.pone.0226486. eCollection 2020. PLoS One. 2020. PMID: 31931515 Free PMC article.
-
Antidepressant effects of combination of brexpiprazole and fluoxetine on depression-like behavior and dendritic changes in mice after inflammation.Psychopharmacology (Berl). 2017 Feb;234(4):525-533. doi: 10.1007/s00213-016-4483-7. Epub 2016 Nov 15. Psychopharmacology (Berl). 2017. PMID: 27844095 Free PMC article.
-
Second Generation Antipsychotics in the Treatment of Major Depressive Disorder: An Update.Chonnam Med J. 2016 Sep;52(3):159-72. doi: 10.4068/cmj.2016.52.3.159. Epub 2016 Sep 23. Chonnam Med J. 2016. PMID: 27689026 Free PMC article. Review.
-
Alterations in amino acid levels in mouse brain regions after adjunctive treatment of brexpiprazole with fluoxetine: comparison with (R)-ketamine.Psychopharmacology (Berl). 2017 Nov;234(21):3165-3173. doi: 10.1007/s00213-017-4700-z. Epub 2017 Jul 26. Psychopharmacology (Berl). 2017. PMID: 28748374
-
Antidepressant functions of Jie Yu Chu Fan capsule in promoting hippocampal nerve cell neurogenesis in a mouse model of chronic unpredictable mild stress.Ann Transl Med. 2020 Aug;8(16):1020. doi: 10.21037/atm-20-5599. Ann Transl Med. 2020. PMID: 32953820 Free PMC article.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association: Washington, DC, USA; 2000.
-
- American Psychiatric Association 2010Practice Guideline for the Treatment of Patients with Major Depressive DisorderThird EditionAmerican Psychiatric Association: Washington, DC, USA
-
- Andersen SW, Clemow DB, Corya SA. Long-term weight gain in patients treated with open-label olanzapine in combination with fluoxetine for major depressive disorder. J Clin Psychiatry. 2005;66:1468–1476. - PubMed
-
- Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2008;22:343–396. - PubMed
-
- Baldomero EB, Ubago JG, Cercos CL, Ruiloba JV, Calvo CG, Lopez RP. Venlafaxine extended release versus conventional antidepressants in the remission of depressive disorders after previous antidepressant failure: ARGOS study. Depress Anxiety. 2005;22:68–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

