Characterization of the deoxyguanosine-lysine cross-link of methylglyoxal
- PMID: 24801980
- PMCID: PMC4060920
- DOI: 10.1021/tx500068v
Characterization of the deoxyguanosine-lysine cross-link of methylglyoxal
Abstract
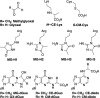
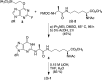
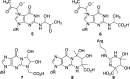
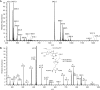
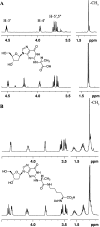
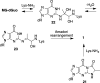
Methylglyoxal is a mutagenic bis-electrophile that is produced endogenously from carbohydrate precursors. Methylglyoxal has been reported to induce DNA-protein cross-links (DPCs) in vitro and in cultured cells. Previous work suggests that these cross-links are formed between guanine and either lysine or cysteine side chains. However, the chemical nature of the methylglyoxal induced DPC have not been determined. We have examined the reaction of methylglyoxal, deoxyguanosine (dGuo), and Nα-acetyllysine (AcLys) and determined the structure of the cross-link to be the N2-ethyl-1-carboxamide with the lysine side chain amino group (1). The cross-link was identified by mass spectrometry and the structure confirmed by comparison to a synthetic sample. Further, the cross-link between methylglyoxal, dGuo, and a peptide (AcAVAGKAGAR) was also characterized. The mechanism of cross-link formation is likely to involve an Amadori rearrangement.
Figures






Similar articles
-
Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal.J Biol Chem. 1996 Aug 9;271(32):19338-45. doi: 10.1074/jbc.271.32.19338. J Biol Chem. 1996. PMID: 8702619
-
N2,7-bis(1-hydroxy-2-oxopropyl)-2'-deoxyguanosine: identical noncyclic adducts with 1,3-dichloropropene epoxides and methylglyoxal.Chem Res Toxicol. 1998 Dec;11(12):1536-42. doi: 10.1021/tx9801256. Chem Res Toxicol. 1998. PMID: 9860499
-
DNA-protein cross-links between guanine and lysine depend on the mechanism of oxidation for formation of C5 vs C8 guanosine adducts.J Am Chem Soc. 2008 Jan 16;130(2):703-9. doi: 10.1021/ja077102a. J Am Chem Soc. 2008. PMID: 18081286
-
Synthesis of G-N2-(CH2)3-N2-G Trimethylene DNA Interstrand Cross-Links.Curr Protoc Nucleic Acid Chem. 2014 Mar 26;56:5.14.1-15. doi: 10.1002/0471142700.nc0514s56. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25606979 Review.
-
Mechanisms of DNA-protein cross-link formation and repair.Biochim Biophys Acta Proteins Proteom. 2021 Aug;1869(8):140669. doi: 10.1016/j.bbapap.2021.140669. Epub 2021 May 3. Biochim Biophys Acta Proteins Proteom. 2021. PMID: 33957291 Review.
Cited by
-
Aldehyde-induced DNA-protein crosslinks- DNA damage, repair and mutagenesis.Front Oncol. 2024 Sep 12;14:1478373. doi: 10.3389/fonc.2024.1478373. eCollection 2024. Front Oncol. 2024. PMID: 39328207 Free PMC article. Review.
-
Endogenous Cellular Metabolite Methylglyoxal Induces DNA-Protein Cross-Links in Living Cells.ACS Chem Biol. 2024 Jun 21;19(6):1291-1302. doi: 10.1021/acschembio.4c00100. Epub 2024 May 16. ACS Chem Biol. 2024. PMID: 38752800 Free PMC article.
-
Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease.Chem Res Toxicol. 2022 Oct 17;35(10):1720-1746. doi: 10.1021/acs.chemrestox.2c00160. Epub 2022 Oct 5. Chem Res Toxicol. 2022. PMID: 36197742 Free PMC article. Review.
-
Methylglyoxal mutagenizes single-stranded DNA via Rev1-associated slippage and mispairing.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf705. doi: 10.1093/nar/gkaf705. Nucleic Acids Res. 2025. PMID: 40705923 Free PMC article.
-
5'-O-Alkylpyridoxamines: Lipophilic Analogues of Pyridoxamine Are Potent Scavengers of 1,2-Dicarbonyls.Chem Res Toxicol. 2015 Jul 20;28(7):1469-75. doi: 10.1021/acs.chemrestox.5b00148. Epub 2015 Jun 17. Chem Res Toxicol. 2015. PMID: 26046387 Free PMC article.
References
-
- Thornalley P. J. (1996) Pharmacology of methylglyoxal: Formation, modification of proteins and nucleic acids, and enzymatic detoxification - a role in pathogenesis and antiproliferative chemotherapy. Gen. Pharmacol. 27, 565–573. - PubMed
-
- Nemet I.; Varga-Defterdarović L.; Turk Z. (2006) Methylglyoxal in food and living organisms. Mol. Nutr. Food Res. 50, 1105–1117. - PubMed
-
- Kalapos M. P. (2013) Where does plasma methylglyoxal originate from?. Diabetes Res. Clin. Pract. 99, 260–271. - PubMed
-
- Singh R.; Barden A.; Mori T.; Beilin L. (2001) Advanced glycation end-products: A review. Diabetologia 44, 129–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

