Improved cyclopropanation activity of histidine-ligated cytochrome P450 enables the enantioselective formal synthesis of levomilnacipran
- PMID: 24802161
- PMCID: PMC4120663
- DOI: 10.1002/anie.201402809
Improved cyclopropanation activity of histidine-ligated cytochrome P450 enables the enantioselective formal synthesis of levomilnacipran
Abstract
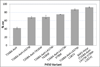
Engineering enzymes capable of modes of activation unprecedented in nature will increase the range of industrially important molecules that can be synthesized through biocatalysis. However, low activity for a new function is often a limitation in adopting enzymes for preparative-scale synthesis, reaction with demanding substrates, or when a natural substrate is also present. By mutating the proximal ligand and other key active-site residues of the cytochrome P450 enzyme from Bacillus megaterium (P450-BM3), a highly active His-ligated variant of P450-BM3 that can be employed for the enantioselective synthesis of the levomilnacipran core was engineered. This enzyme, BM3-Hstar, catalyzes the cyclopropanation of N,N-diethyl-2-phenylacrylamide with an estimated initial rate of over 1000 turnovers per minute and can be used under aerobic conditions. Cyclopropanation activity is highly dependent on the electronic properties of the P450 proximal ligand, which can be used to tune this non-natural enzyme activity.
Keywords: biocatalysis; cyclopropanation; cytochrome P450; enzymes; protein engineering.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
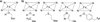


References
-
- Huisman GW, Collier SJ. Curr. Op. in Biotech. 2013;17:284–292. - PubMed
-
- Wohlgemuth R. Curr. Op. in Biotech. 2010;21:713–724. - PubMed
-
-
For reviews, see: Lebel H, Marcoux J-F, Molinaro C, Charette AB. Chem. Rev. 2003;103:977–1050. Doyle M, Forbes DC. Chem. Rev. 1998;98:911–935. For selected examples, see: Aratani T. Pure & Appl. Chem. 1985;57:1839–1844. Evans D, Woerpel KA, Hinman MA, Faul MM. J. Am. Chem. Soc. 1991;113:726–728. Davies HM, Venkataramani C. Org. Lett. 2003;5:1403–1406. For recent examples, see: Marcoux D, Azzi S, Charette AB. J. Am. Chem. Soc. 2009;131:6970–6972. Qin C, Boyarskikh V, Hansen JH, Hardcastle KI, Musaev DG, Davies HML. J. Am. Chem. Soc. 2011;133:19198–19204.
-
-
- Purkarthofer T, Gruber K, Gruber-Khadjawi M, Waich K, Mink D, Griengl H. Angew. Chem. Int. Ed. 2006;45:3454–3456. - PubMed
- Johnson DV, Zabelinskaja-Mackova AA, Griengl H. Curr. Op. Chem. Bio. 2000;4:103–109. - PubMed
- McLoughlin SY, Copley SD. Proc. Nat. Aca. Sci. 2008;105:13497–13502. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

