Tualang honey improves human corneal epithelial progenitor cell migration and cellular resistance to oxidative stress in vitro
- PMID: 24802273
- PMCID: PMC4011849
- DOI: 10.1371/journal.pone.0096800
Tualang honey improves human corneal epithelial progenitor cell migration and cellular resistance to oxidative stress in vitro
Erratum in
- PLoS One. 2014;9(8):e105233
Abstract
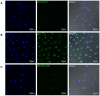
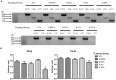
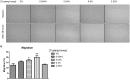
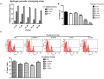
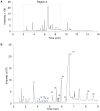
Stem cells with enhanced resistance to oxidative stress after in vitro expansion have been shown to have improved engraftment and regenerative capacities. Such cells can be generated by preconditioning them with exposure to an antioxidant. In this study we evaluated the effects of Tualang honey (TH), an antioxidant-containing honey, on human corneal epithelial progenitor (HCEP) cells in culture. Cytotoxicity, gene expression, migration, and cellular resistance to oxidative stress were evaluated. Immunofluorescence staining revealed that HCEP cells were holoclonal and expressed epithelial stem cell marker p63 without corneal cytokeratin 3. Cell viability remained unchanged after cells were cultured with 0.004, 0.04, and 0.4% TH in the medium, but it was significantly reduced when the concentration was increased to 3.33%. Cell migration, tested using scratch migration assay, was significantly enhanced when cells were cultured with TH at 0.04% and 0.4%. We also found that TH has hydrogen peroxide (H2O2) scavenging ability, although a trace level of H2O2 was detected in the honey in its native form. Preconditioning HCEP cells with 0.4% TH for 48 h showed better survival following H2O2-induced oxidative stress at 50 µM than untreated group, with a significantly lower number of dead cells (15.3 ± 0.4%) were observed compared to the untreated population (20.5 ± 0.9%, p<0.01). Both TH and ascorbic acid improved HCEP viability following induction of 100 µM H2O2, but the benefit was greater with TH treatment than with ascorbic acid. However, no significant advantage was demonstrated using 5-hydroxymethyl-2-furancarboxaldehyde, a compound that was found abundant in TH using GC/MS analysis. This suggests that the cellular anti-oxidative capacity in HCEP cells was augmented by native TH and was attributed to its antioxidant properties. In conclusion, TH possesses antioxidant properties and can improve cell migration and cellular resistance to oxidative stress in HCEP cells in vitro.
Conflict of interest statement
Figures

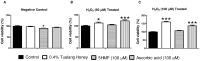
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

