Simplifying electronic data capture in clinical trials: workflow embedded image and biosignal file integration and analysis via web services
- PMID: 24802371
- PMCID: PMC4171435
- DOI: 10.1007/s10278-014-9694-z
Simplifying electronic data capture in clinical trials: workflow embedded image and biosignal file integration and analysis via web services
Abstract
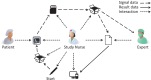
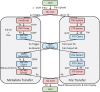
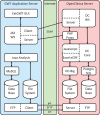
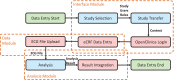
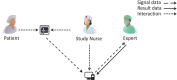
To improve data quality and save cost, clinical trials are nowadays performed using electronic data capture systems (EDCS) providing electronic case report forms (eCRF) instead of paper-based CRFs. However, such EDCS are insufficiently integrated into the medical workflow and lack in interfacing with other study-related systems. In addition, most EDCS are unable to handle image and biosignal data, although electrocardiography (EGC, as example for one-dimensional (1D) data), ultrasound (2D data), or magnetic resonance imaging (3D data) have been established as surrogate endpoints in clinical trials. In this paper, an integrated workflow based on OpenClinica, one of the world's largest EDCS, is presented. Our approach consists of three components for (i) sharing of study metadata, (ii) integration of large volume data into eCRFs, and (iii) automatic image and biosignal analysis. In all components, metadata is transferred between systems using web services and JavaScript, and binary large objects (BLOBs) are sent via the secure file transfer protocol and hypertext transfer protocol. We applied the close-looped workflow in a multicenter study, where long term (7 days/24 h) Holter ECG monitoring is acquired on subjects with diabetes. Study metadata is automatically transferred into OpenClinica, the 4 GB BLOBs are seamlessly integrated into the eCRF, automatically processed, and the results of signal analysis are written back into the eCRF immediately.
Figures
References
-
- Leroux H, McBride S, Gibson S. On selecting a clinical trial management system for large scale, multicenter, multi-modal clinical research study. Stud Health Technol Inform. 2011;168:89–95. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

