Survival outcome of early versus delayed bevacizumab treatment in patients with recurrent glioblastoma
- PMID: 24803001
- PMCID: PMC4297475
- DOI: 10.1007/s11060-014-1460-z
Survival outcome of early versus delayed bevacizumab treatment in patients with recurrent glioblastoma
Abstract
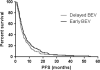
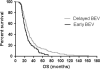
Bevacizumab (BEV) is widely used for treatment of patients with recurrent glioblastoma. It is not known if there are differences in outcome between early versus delayed BEV treatment of recurrent glioblastoma. We examined the relationship between the time of starting BEV treatment and outcomes in patients with recurrent glioblastoma. In this retrospective chart review, we identified patients with recurrent glioblastoma diagnosed between 2005 and 2011 who were treated with BEV alone or BEV-containing regimens. Data was analyzed to determine overall survival (OS) from time of diagnosis and progression free survival (PFS) from time of starting BEV. A total of 298 patients were identified, 112 patients received early BEV, 133 patients received delayed BEV, and 53 patients were excluded because they either progressed within 3 months of radiation or received BEV at the time of diagnosis. There was no significant difference in PFS between patients that received early BEV and those that received delayed BEV (5.2 vs. 4.3 months, p = 0.2). Patients treated with delayed BEV had longer OS when compared to those treated with early BEV (25.9 vs. 20.8 months, p = 0.005). In patients with recurrent glioblastoma, there was no significant difference in PFS from the time of starting BEV between early and delayed BEV. Although patients treated with delayed BEV seemed to have longer OS, a conclusion regarding OS outcome requires further prospective trials. These results may indicate that delaying treatment with BEV is not detrimental for survival of patients with recurrent glioblastoma.
Figures
References
-
- Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. doi:10.1634/theoncologist.2009-0121.Epub. - PubMed
-
- Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical