The impact of intestinal inflammation on the nutritional environment of the gut microbiota
- PMID: 24803011
- PMCID: PMC4219934
- DOI: 10.1016/j.imlet.2014.04.014
The impact of intestinal inflammation on the nutritional environment of the gut microbiota
Abstract
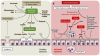
The intestinal epithelium is a single cell barrier separating a sterile mucosal tissue from a large microbial community dominated by obligate anaerobic bacteria, which inhabit the gut lumen. To maintain mucosal integrity, any breach in the epithelial barrier needs to be met with an inflammatory host response designed to repel microbial intruders from the tissue, protect the mucosal surface and repair injuries to the epithelium. In addition, inflammation induces mechanisms of nutritional immunity, which limit the availability of metals in the intestinal lumen, thereby imposing new selective forces on microbial growth. However, the inflammatory host response also has important side effects. A by-product of producing reactive oxygen and nitrogen species aimed at eradicating microbial intruders is the luminal generation of exogenous electron acceptors. The presence of these electron acceptors creates a new metabolic niche that is filled by facultative anaerobic bacteria. Here we review the changes in microbial nutrient utilization that accompany intestinal inflammation and the consequent changes in the composition of gut-associated microbial communities.
Keywords: Anaerobic respiration; Intestinal inflammation; Microbiota; Nutritional immunity.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
References
-
- Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS letters. 2005;579:4911–4917. - PubMed
-
- Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. American journal of physiology Cell physiology. 2006;290:C433–443. - PubMed
-
- Salzman A, Denenberg AG, Ueta I, O’Connor M, Linn SC, Szabo C. Induction and activity of nitric oxide synthase in cultured human intestinal epithelial monolayers. The American journal of physiology. 1996;270:G565–573. - PubMed
-
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. - PubMed
-
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

