Leucophores are similar to xanthophores in their specification and differentiation processes in medaka
- PMID: 24803434
- PMCID: PMC4034200
- DOI: 10.1073/pnas.1311254111
Leucophores are similar to xanthophores in their specification and differentiation processes in medaka
Abstract
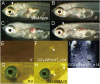
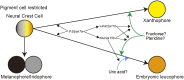
Animal body color is generated primarily by neural crest-derived pigment cells in the skin. Mammals and birds have only melanocytes on the surface of their bodies; however, fish have a variety of pigment cell types or chromatophores, including melanophores, xanthophores, and iridophores. The medaka has a unique chromatophore type called the leucophore. The genetic basis of chromatophore diversity remains poorly understood. Here, we report that three loci in medaka, namely, leucophore free (lf), lf-2, and white leucophore (wl), which affect leucophore and xanthophore differentiation, encode solute carrier family 2, member 15b (slc2a15b), paired box gene 7a (pax7a), and solute carrier family 2 facilitated glucose transporter, member 11b (slc2a11b), respectively. Because lf-2, a loss-of-function mutant for pax7a, causes defects in the formation of xanthophore and leucophore precursor cells, pax7a is critical for the development of the chromatophores. This genetic evidence implies that leucophores are similar to xanthophores, although it was previously thought that leucophores were related to iridophores, as these chromatophores have purine-dependent light reflection. Our identification of slc2a15b and slc2a11b as genes critical for the differentiation of leucophores and xanthophores in medaka led to a further finding that the existence of these two genes in the genome coincides with the presence of xanthophores in nonmammalian vertebrates: birds have yellow-pigmented irises with xanthophore-like intracellular organelles. Our findings provide clues for revealing diverse evolutionary mechanisms of pigment cell formation in animals.
Keywords: genome evolution; neural crest differentiation; pigment cell variation; vertebrate body color.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Leucophore identity is more gold than silver.Pigment Cell Melanoma Res. 2015 Mar;28(2):131. doi: 10.1111/pcmr.12349. Epub 2015 Feb 9. Pigment Cell Melanoma Res. 2015. PMID: 25546351 No abstract available.
References
-
- Fujii R. Cytophysiology of fish chromatophores. Int Rev Cytol. 1993;143:191–255.
-
- Fujii R. The regulation of motile activity in fish chromatophores. Pigment Cell Res. 2000;13(5):300–319. - PubMed
-
- Hama T. Chromatophores and iridocytes. In: Yamamoto T, editor. MEDAKA (KILLIFISH) Biology and Strains. Tokyo: Keigaku; 1975. pp. 138–153.
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

