Mycobacterial Esx-3 requires multiple components for iron acquisition
- PMID: 24803520
- PMCID: PMC4010830
- DOI: 10.1128/mBio.01073-14
Mycobacterial Esx-3 requires multiple components for iron acquisition
Abstract
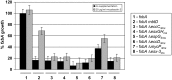
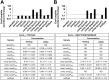
ABSTRACT The type VII secretion systems are conserved across mycobacterial species and in many Gram-positive bacteria. While the well-characterized Esx-1 pathway is required for the virulence of pathogenic mycobacteria and conjugation in the model organism Mycobacterium smegmatis, Esx-3 contributes to mycobactin-mediated iron acquisition in these bacteria. Here we show that several Esx-3 components are individually required for function under low-iron conditions but that at least one, the membrane-bound protease MycP3 of M. smegmatis, is partially expendable. All of the esx-3 mutants tested, including the ΔmycP3ms mutant, failed to export the native Esx-3 substrates EsxHms and EsxGms to quantifiable levels, as determined by targeted mass spectrometry. Although we were able to restore low-iron growth to the esx-3 mutants by genetic complementation, we found a wide range of complementation levels for protein export. Indeed, minute quantities of extracellular EsxHms and EsxGms were sufficient for iron acquisition under our experimental conditions. The apparent separation of Esx-3 function in iron acquisition from robust EsxGms and EsxHms secretion in the ΔmycP3ms mutant and in some of the complemented esx-3 mutants compels reexamination of the structure-function relationships for type VII secretion systems. IMPORTANCE Mycobacteria have several paralogous type VII secretion systems, Esx-1 through Esx-5. Whereas Esx-1 is required for pathogenic mycobacteria to grow within an infected host, Esx-3 is essential for growth in vitro. We and others have shown that Esx-3 is required for siderophore-mediated iron acquisition. In this work, we identify individual Esx-3 components that contribute to this process. As in the Esx-1 system, most mutations that abolish Esx-3 protein export also disrupt its function. Unexpectedly, however, ultrasensitive quantitation of Esx-3 secretion by multiple-reaction-monitoring mass spectrometry (MRM-MS) revealed that very low levels of export were sufficient for iron acquisition under similar conditions. Although protein export clearly contributes to type VII function, the relationship is not absolute.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
