Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1
- PMID: 24803522
- PMCID: PMC4010835
- DOI: 10.1128/mBio.01130-14
Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1
Abstract
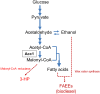
ABSTRACT Acetyl coenzyme A (acetyl-CoA) carboxylase (ACCase) plays a central role in carbon metabolism and has been the site of action for the development of therapeutics or herbicides, as its product, malonyl-CoA, is a precursor for production of fatty acids and other compounds. Control of Acc1 activity in the yeast Saccharomyces cerevisiae occurs mainly at two levels, i.e., regulation of transcription and repression by Snf1 protein kinase at the protein level. Here, we demonstrate a strategy for improving the activity of ACCase in S. cerevisiae by abolishing posttranslational regulation of Acc1 via site-directed mutagenesis. It was found that introduction of two site mutations in Acc1, Ser659 and Ser1157, resulted in an enhanced activity of Acc1 and increased total fatty acid content. As Snf1 regulation of Acc1 is particularly active under glucose-limited conditions, we evaluated the effect of the two site mutations in chemostat cultures. Finally, we showed that our modifications of Acc1 could enhance the supply of malonyl-CoA and therefore successfully increase the production of two industrially important products derived from malonyl-CoA, fatty acid ethyl esters and 3-hydroxypropionic acid. IMPORTANCE ACCase is responsible for carboxylation of acetyl-CoA to produce malonyl-CoA, which is a crucial step in the control of fatty acid metabolism. ACCase opened the door for pharmaceutical treatments of obesity and diabetes as well as the development of new herbicides. ACCase is also recognized as a promising target for developing cell factories, as its malonyl-CoA product serves as a universal precursor for a variety of high-value compounds in white biotechnology. Yeast ACCase is a good model in understanding the enzyme's catalysis, regulation, and inhibition. The present study describes the importance of protein phosphorylation in regulation of yeast ACCase and identifies potential regulation sites. This study led to the generation of a more efficient ACCase, which was applied in the production of two high-value compounds derived from malonyl-CoA, i.e., fatty acid ethyl esters that can be used as biodiesel and 3-hydroxypropionic acid that is considered an important platform chemical.
Figures

Similar articles
-
Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis.Appl Microbiol Biotechnol. 2020 Jul;104(14):6057-6065. doi: 10.1007/s00253-020-10643-7. Epub 2020 May 8. Appl Microbiol Biotechnol. 2020. PMID: 32385515 Free PMC article. Review.
-
Screening Phosphorylation Site Mutations in Yeast Acetyl-CoA Carboxylase Using Malonyl-CoA Sensor to Improve Malonyl-CoA-Derived Product.Front Microbiol. 2018 Jan 25;9:47. doi: 10.3389/fmicb.2018.00047. eCollection 2018. Front Microbiol. 2018. PMID: 29422886 Free PMC article.
-
Improving polyketide and fatty acid synthesis by engineering of the yeast acetyl-CoA carboxylase.J Biotechnol. 2014 Oct 10;187:56-9. doi: 10.1016/j.jbiotec.2014.07.430. Epub 2014 Jul 29. J Biotechnol. 2014. PMID: 25078432
-
Acetyl-CoA carboxylase 1-dependent lipogenesis promotes autophagy downstream of AMPK.J Biol Chem. 2019 Aug 9;294(32):12020-12039. doi: 10.1074/jbc.RA118.007020. Epub 2019 Jun 17. J Biol Chem. 2019. PMID: 31209110 Free PMC article.
-
Malonyl CoA control of fatty acid oxidation in the ischemic heart.J Mol Cell Cardiol. 2002 Sep;34(9):1099-109. doi: 10.1006/jmcc.2002.2060. J Mol Cell Cardiol. 2002. PMID: 12392882 Review.
Cited by
-
Improved ethyl caproate production of Chinese liquor yeast by overexpressing fatty acid synthesis genes with OPI1 deletion.J Ind Microbiol Biotechnol. 2016 Sep;43(9):1261-70. doi: 10.1007/s10295-016-1795-x. Epub 2016 Jun 25. J Ind Microbiol Biotechnol. 2016. PMID: 27344573
-
Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories.Nat Commun. 2016 May 25;7:11709. doi: 10.1038/ncomms11709. Nat Commun. 2016. PMID: 27222209 Free PMC article.
-
Engineering Plant Secondary Metabolism in Microbial Systems.Plant Physiol. 2019 Mar;179(3):844-861. doi: 10.1104/pp.18.01291. Epub 2019 Jan 14. Plant Physiol. 2019. PMID: 30643013 Free PMC article.
-
Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis.Appl Microbiol Biotechnol. 2020 Jul;104(14):6057-6065. doi: 10.1007/s00253-020-10643-7. Epub 2020 May 8. Appl Microbiol Biotechnol. 2020. PMID: 32385515 Free PMC article. Review.
-
Developments in Fatty Acid-Derived Insect Pheromone Production Using Engineered Yeasts.Front Microbiol. 2021 Nov 11;12:759975. doi: 10.3389/fmicb.2021.759975. eCollection 2021. Front Microbiol. 2021. PMID: 34858372 Free PMC article. Review.
References
-
- Podkowinski J, Tworak A. 2011. Acetyl-coenzyme A carboxylase—an attractive enzyme for biotechnology. Biotechnologia 92:321–335. 10.1007/s00018-005-5121-4 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
