Apical constriction: themes and variations on a cellular mechanism driving morphogenesis
- PMID: 24803648
- PMCID: PMC4011084
- DOI: 10.1242/dev.102228
Apical constriction: themes and variations on a cellular mechanism driving morphogenesis
Abstract
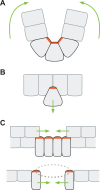
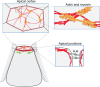
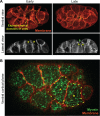
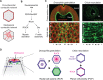
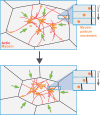
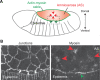
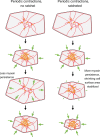
Apical constriction is a cell shape change that promotes tissue remodeling in a variety of homeostatic and developmental contexts, including gastrulation in many organisms and neural tube formation in vertebrates. In recent years, progress has been made towards understanding how the distinct cell biological processes that together drive apical constriction are coordinated. These processes include the contraction of actin-myosin networks, which generates force, and the attachment of actin networks to cell-cell junctions, which allows forces to be transmitted between cells. Different cell types regulate contractility and adhesion in unique ways, resulting in apical constriction with varying dynamics and subcellular organizations, as well as a variety of resulting tissue shape changes. Understanding both the common themes and the variations in apical constriction mechanisms promises to provide insight into the mechanics that underlie tissue morphogenesis.
Keywords: Actin; Adhesion; Apical; Cadherin; Constriction; Myosin.
Figures
Similar articles
-
Apical constriction: a cell shape change that can drive morphogenesis.Dev Biol. 2010 May 1;341(1):5-19. doi: 10.1016/j.ydbio.2009.09.009. Epub 2009 Sep 12. Dev Biol. 2010. PMID: 19751720 Free PMC article. Review.
-
Eph-Ephrin signaling and focal adhesion kinase regulate actomyosin-dependent apical constriction of ciliary band cells.Development. 2014 Mar;141(5):1075-84. doi: 10.1242/dev.100123. Development. 2014. PMID: 24550115
-
The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila.Development. 2010 May;137(10):1645-55. doi: 10.1242/dev.044107. Epub 2010 Apr 14. Development. 2010. PMID: 20392741
-
Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells.Dev Biol. 2007 Nov 1;311(1):40-52. doi: 10.1016/j.ydbio.2007.08.010. Epub 2007 Aug 10. Dev Biol. 2007. PMID: 17868669 Free PMC article.
-
Caenorhabditis elegans Gastrulation: A Model for Understanding How Cells Polarize, Change Shape, and Journey Toward the Center of an Embryo.Genetics. 2020 Feb;214(2):265-277. doi: 10.1534/genetics.119.300240. Genetics. 2020. PMID: 32029580 Free PMC article. Review.
Cited by
-
Differential cellular stiffness across tissues that contribute to Xenopus neural tube closure.Dev Growth Differ. 2024 Jun;66(5):320-328. doi: 10.1111/dgd.12936. Epub 2024 Jun 26. Dev Growth Differ. 2024. PMID: 38925637 Free PMC article.
-
Canonical and Non-Canonical Localization of Tight Junction Proteins during Early Murine Cranial Development.Int J Mol Sci. 2024 Jan 24;25(3):1426. doi: 10.3390/ijms25031426. Int J Mol Sci. 2024. PMID: 38338705 Free PMC article.
-
The Drosophila melanogaster Rab GAP RN-tre cross-talks with the Rho1 signaling pathway to regulate nonmuscle myosin II localization and function.Mol Biol Cell. 2020 Oct 1;31(21):2379-2397. doi: 10.1091/mbc.E20-03-0181. Epub 2020 Aug 20. Mol Biol Cell. 2020. PMID: 32816624 Free PMC article.
-
The cellular dynamics of neural tube formation.Biochem Soc Trans. 2023 Feb 27;51(1):343-352. doi: 10.1042/BST20220871. Biochem Soc Trans. 2023. PMID: 36794768 Free PMC article.
-
The RhoGEF protein Plekhg5 regulates apical constriction of bottle cells during gastrulation.Development. 2018 Dec 12;145(24):dev168922. doi: 10.1242/dev.168922. Development. 2018. PMID: 30446627 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

