Iron binding capacity of dephytinised soy protein isolate hydrolysate as influenced by the degree of hydrolysis and enzyme type
- PMID: 24803710
- PMCID: PMC4008747
- DOI: 10.1007/s13197-011-0586-7
Iron binding capacity of dephytinised soy protein isolate hydrolysate as influenced by the degree of hydrolysis and enzyme type
Abstract
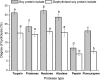
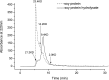
Soy protein is increasingly used in extended meat products and dairy type products due to the presence of high quality proteins with excellent functional properties. However, it has been shown to inhibit iron bioavailability because of phytic acid present in the protein. This present study investigated the effects of dephytinise from soy protein isolate (SPI) on iron binding capacity and degree of hydrolysis. Also the effects of enzyme type and degree of hydrolysis on iron binding capacity were studied. It was demonstrated that phytase and anion exchange resin could remove effectively the phytate from SPI. The dephytinise would decrease the degree of hydrolysis of SPI. The enzyme type and degree of hydrolysis influenced significantly the iron binding capacity of the hydrolysate. Flavourzyme might be the best choice for producing peptides with iron binding capacity from SPI and middle degree of hydrolysis would be benefitable to this process.
Keywords: Hydrolysis; Iron binding capacity; Phytate; Soy protein isolate.
Figures

Similar articles
-
Effects of phytase-assisted processing method on physicochemical and functional properties of soy protein isolate.J Agric Food Chem. 2014 Nov 12;62(45):10989-97. doi: 10.1021/jf503952s. Epub 2014 Nov 3. J Agric Food Chem. 2014. PMID: 25333697
-
Differential Enzymatic Hydrolysis: A Study on Its Impact on Soy Protein Structure, Function, and Soy Milk Powder Properties.Foods. 2025 Mar 6;14(5):906. doi: 10.3390/foods14050906. Foods. 2025. PMID: 40077608 Free PMC article.
-
Physicochemical properties of soy protein hydrolysate and its formulation and stability with encapsulated probiotic under in vitro gastrointestinal environment.J Food Sci. 2020 Oct;85(10):3543-3551. doi: 10.1111/1750-3841.15399. Epub 2020 Aug 31. J Food Sci. 2020. PMID: 32869300
-
The effect of food processing on phytate hydrolysis and availability of iron and zinc.Adv Exp Med Biol. 1991;289:499-508. doi: 10.1007/978-1-4899-2626-5_33. Adv Exp Med Biol. 1991. PMID: 1654732 Review.
-
Phytic acid interactions in food systems.Crit Rev Food Sci Nutr. 1980;13(4):297-335. doi: 10.1080/10408398009527293. Crit Rev Food Sci Nutr. 1980. PMID: 7002470 Review.
Cited by
-
Autonomous Defense Based on Biogenic Nanoparticle Formation in Daunomycin-Producing Streptomyces.Microorganisms. 2025 Jan 8;13(1):107. doi: 10.3390/microorganisms13010107. Microorganisms. 2025. PMID: 39858875 Free PMC article.
-
Screening for Metal-Chelating Activity in Potato Protein Hydrolysates Using Surface Plasmon Resonance and Peptidomics.Antioxidants (Basel). 2024 Mar 13;13(3):346. doi: 10.3390/antiox13030346. Antioxidants (Basel). 2024. PMID: 38539879 Free PMC article.
-
Antioxidant activities and functional properties of enzymatic protein hydrolysates from defatted Camellia oleifera seed cake.J Food Sci Technol. 2015 Sep;52(9):5681-90. doi: 10.1007/s13197-014-1693-z. Epub 2015 Jan 8. J Food Sci Technol. 2015. PMID: 26344981 Free PMC article.
-
Protein Hydrolysates as Promoters of Non-Haem Iron Absorption.Nutrients. 2017 Jun 15;9(6):609. doi: 10.3390/nu9060609. Nutrients. 2017. PMID: 28617327 Free PMC article. Review.
References
-
- Bao XL, Song M, Zhang J, Yang C, Guo ST. Calcium-binding ability of soy protein hydrolysates. Chin Chem Lett. 2007;18:1115–1118. doi: 10.1016/j.cclet.2007.07.032. - DOI
-
- Brooks JR, Morr CV. Phytate removal from soy protein isolates using ion exchange processing treatments. J Food Sci. 1982;47:1280–1282. doi: 10.1111/j.1365-2621.1982.tb07666.x. - DOI
-
- Carroll KK. Review of clinical studies on cholesterol lowing response to soy protein. Am J Diet Assoc. 1991;91:820–827. - PubMed
-
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. - DOI
LinkOut - more resources
Full Text Sources
