Cervical dystonia: a disorder of the midbrain network for covert attentional orienting
- PMID: 24803911
- PMCID: PMC4009446
- DOI: 10.3389/fneur.2014.00054
Cervical dystonia: a disorder of the midbrain network for covert attentional orienting
Abstract
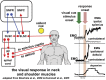
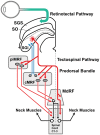
While the pathogenesis of cervical dystonia remains unknown, recent animal and clinical experimental studies have indicated its probable mechanisms. Abnormal temporal discrimination is a mediational endophenotype of cervical dystonia and informs new concepts of disease pathogenesis. Our hypothesis is that both abnormal temporal discrimination and cervical dystonia are due to a disorder of the midbrain network for covert attentional orienting caused by reduced gamma-aminobutyric acid (GABA) inhibition, resulting, in turn, from as yet undetermined, genetic mutations. Such disinhibition is (a) subclinically manifested by abnormal temporal discrimination due to prolonged duration firing of the visual sensory neurons in the superficial laminae of the superior colliculus and (b) clinically manifested by cervical dystonia due to disinhibited burst activity of the cephalomotor neurons of the intermediate and deep laminae of the superior colliculus. Abnormal temporal discrimination in unaffected first-degree relatives of patients with cervical dystonia represents a subclinical manifestation of defective GABA activity both within the superior colliculus and from the substantia nigra pars reticulata. A number of experiments are required to prove or disprove this hypothesis.
Keywords: GABA; cervical dystonia; covert attention; superior colliculus; temporal discrimination.
Figures

Similar articles
-
Temporal Discrimination: Mechanisms and Relevance to Adult-Onset Dystonia.Front Neurol. 2017 Nov 28;8:625. doi: 10.3389/fneur.2017.00625. eCollection 2017. Front Neurol. 2017. PMID: 29234300 Free PMC article. Review.
-
Disrupted superior collicular activity may reveal cervical dystonia disease pathomechanisms.Sci Rep. 2017 Dec 1;7(1):16753. doi: 10.1038/s41598-017-17074-x. Sci Rep. 2017. PMID: 29196716 Free PMC article.
-
A Study of the Midbrain Network for Covert Attentional Orienting in Cervical Dystonia Patients using Dynamic Causal Modelling.Annu Int Conf IEEE Eng Med Biol Soc. 2019 Jul;2019:3519-3522. doi: 10.1109/EMBC.2019.8857152. Annu Int Conf IEEE Eng Med Biol Soc. 2019. PMID: 31946637
-
Temporal discrimination, a cervical dystonia endophenotype: penetrance and functional correlates.Mov Disord. 2014 May;29(6):804-11. doi: 10.1002/mds.25822. Epub 2014 Jan 30. Mov Disord. 2014. PMID: 24482092
-
GABAergic control of substantia nigra dopaminergic neurons.Prog Brain Res. 2007;160:189-208. doi: 10.1016/S0079-6123(06)60011-3. Prog Brain Res. 2007. PMID: 17499115 Review.
Cited by
-
Young Women do it Better: Sexual Dimorphism in Temporal Discrimination.Front Neurol. 2015 Jul 9;6:160. doi: 10.3389/fneur.2015.00160. eCollection 2015. Front Neurol. 2015. PMID: 26217303 Free PMC article.
-
Sex differences in movement disorders.Nat Rev Neurol. 2020 Feb;16(2):84-96. doi: 10.1038/s41582-019-0294-x. Epub 2020 Jan 3. Nat Rev Neurol. 2020. PMID: 31900464 Review.
-
Autoimmune brainstem encephalitis: Clinical associations, outcomes, and proposed diagnostic criteria.Ann Clin Transl Neurol. 2025 Jan;12(1):213-225. doi: 10.1002/acn3.52273. Epub 2024 Dec 21. Ann Clin Transl Neurol. 2025. PMID: 39708293 Free PMC article.
-
Characterizing the temporal discrimination threshold in musician's dystonia.Sci Rep. 2022 Sep 2;12(1):14939. doi: 10.1038/s41598-022-18739-y. Sci Rep. 2022. PMID: 36056047 Free PMC article.
-
Temporal Discrimination: Mechanisms and Relevance to Adult-Onset Dystonia.Front Neurol. 2017 Nov 28;8:625. doi: 10.3389/fneur.2017.00625. eCollection 2017. Front Neurol. 2017. PMID: 29234300 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

