β-Glucan supplementation, allergy symptoms, and quality of life in self-described ragweed allergy sufferers
- PMID: 24804018
- PMCID: PMC3951572
- DOI: 10.1002/fsn3.11
β-Glucan supplementation, allergy symptoms, and quality of life in self-described ragweed allergy sufferers
Abstract
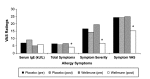
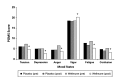
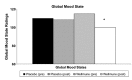
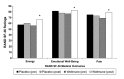
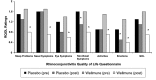
This randomized, placebo-controlled, double-blind study compared the effects of daily supplementation for 4 week with 250 mg Wellmune WGP® β-1,3/1,6-Glucan (WGP) with placebo 250 mg/day (rice flour) on physical and psychological health attributes of self-described "moderate" ragweed allergy sufferers. Study participants (mean age = 36 ± 9 year; range 18-53 year) were recruited before the beginning of ragweed season (September) in Northeastern Ohio. Serum IgE concentration, allergy symptoms [via self-report, Visual Analog Scale (VAS), and Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ)], psychological well-being [Profile of Mood States (POMS)], and physical function (RAND SF-36 Medical Outcomes Study) were measured immediately prior to and after supplementation with WGP (n = 24) or placebo (n = 24) for 4 weeks. Data were analyzed using repeated measures analyses of variance (ANOVA). Compared with placebo, WGP reduced total allergy symptoms (28%), symptom severity (52%), and symptom rating on the VAS (37%) (P < 0.05), but had no effect on serum IgE levels. As measured by the POMS, WGP increased participants' rating of vigor (10%), but reduced tension (34%), depression (45%), anger (41%), fatigue (38%), and confusion (34%) (P < 0.05). Study participants given WGP reported increased physical health (11%), energy (19%), and emotional well-being (7%) compared with study participants given the placebo (RAND SF-36 Medical Outcomes Study). The WGP group also reported decreased sleep problems (53%), reduced nasal symptoms (59%), eye symptoms (57%), non-nasal symptoms (50%), activities (53%), emotions (57%), and improved quality of life (QOL) (56%), as well as improved global mood state (13%). Supplementation with WGP for 4 weeks improved allergy symptoms, overall physical health, and emotional well-being compared with placebo in self-described "moderate" ragweed allergy sufferers during ragweed allergy season.
Keywords: Allergy; IgE; allergy symptom; quality of life; ragweed; rhinitis; β-glucan.
Figures
Similar articles
-
Effect of BETA 1, 3/1, 6 GLUCAN on Upper Respiratory Tract Infection Symptoms and Mood State in Marathon Athletes.J Sports Sci Med. 2009 Dec 1;8(4):509-15. eCollection 2009. J Sports Sci Med. 2009. PMID: 24149590 Free PMC article.
-
Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen.J Allergy Clin Immunol. 2014 Jan;133(1):121-9.e1-2. doi: 10.1016/j.jaci.2013.05.032. Epub 2013 Jul 16. J Allergy Clin Immunol. 2014. PMID: 23870670 Clinical Trial.
-
Factors that affect the allergic rhinitis response to ragweed allergen exposure.Ann Allergy Asthma Immunol. 2010 Apr;104(4):293-8. doi: 10.1016/j.anai.2010.01.012. Ann Allergy Asthma Immunol. 2010. PMID: 20408338
-
Adjuvant therapy with antidepressants for the management of inflammatory bowel disease.Cochrane Database Syst Rev. 2019 Apr 12;4(4):CD012680. doi: 10.1002/14651858.CD012680.pub2. Cochrane Database Syst Rev. 2019. PMID: 30977111 Free PMC article.
-
A review of the clinical efficacy and safety of MP-AzeFlu, a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, in clinical studies conducted during different allergy seasons in the US.J Asthma Allergy. 2016 Jul 11;9:135-43. doi: 10.2147/JAA.S98172. eCollection 2016. J Asthma Allergy. 2016. PMID: 27468241 Free PMC article. Review.
Cited by
-
Effects of β-glucans on fatigue: a systematic review and meta-analysis.Eur J Clin Nutr. 2025 Aug;79(8):705-714. doi: 10.1038/s41430-025-01567-4. Epub 2025 Jan 28. Eur J Clin Nutr. 2025. PMID: 39875626 Free PMC article. Review.
-
Agaricus blazei-Based Mushroom Extract Supplementation to Birch Allergic Blood Donors: A Randomized Clinical Trial.Nutrients. 2019 Oct 2;11(10):2339. doi: 10.3390/nu11102339. Nutrients. 2019. PMID: 31581605 Free PMC article. Clinical Trial.
-
A β-Glucan-Based Dietary Fiber Reduces Mast Cell-Induced Hyperpermeability in Ileum From Patients With Crohn's Disease and Control Subjects.Inflamm Bowel Dis. 2017 Dec 19;24(1):166-178. doi: 10.1093/ibd/izx002. Inflamm Bowel Dis. 2017. PMID: 29272475 Free PMC article.
-
Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials.Molecules. 2019 Mar 30;24(7):1251. doi: 10.3390/molecules24071251. Molecules. 2019. PMID: 30935016 Free PMC article. Review.
-
Cow's milk-based beverage consumption in 1- to 4-year-olds and allergic manifestations: an RCT.Nutr J. 2016 Feb 27;15:19. doi: 10.1186/s12937-016-0138-0. Nutr J. 2016. PMID: 26920136 Free PMC article. Clinical Trial.
References
-
- Adachi Y, Okazaki M, Ohno N, Yadomae T. Enhancement of cytokine production by macrophages stimulated with (1–>3)-b-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol. Pharm. Bull. 1994;17(12):1554–1560. - PubMed
-
- Ahren IL, Williams DL, Rice PJ, Forsgren A, Riesbeck K. The importance of a b-glucan receptor in the nonopsonic entry of nontypeable Haemophilus influenzae into human monocytic and epithelial cells. J. Infect. Dis. 2001;184(2):150–158. - PubMed
-
- Akramiene D, Kondrotas A, Didziapetriene J, Kevelaitis E. Effects of beta-glucans on the immune system. Medicina (Kaunas) 2007;43(8):597–606. - PubMed
-
- Arbes Jr SJ, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2005;116(2):377–383. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

