Biological and functional properties of proteolytic enzyme-modified egg protein by-products
- PMID: 24804027
- PMCID: PMC3967756
- DOI: 10.1002/fsn3.27
Biological and functional properties of proteolytic enzyme-modified egg protein by-products
Abstract
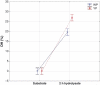
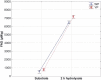
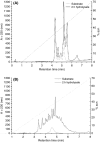
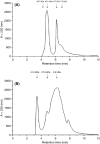
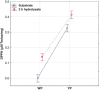
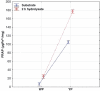
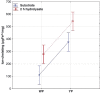
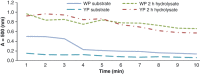
Enzymatic hydrolysis led to improve functional properties and biological activity of protein by-products, which can be further used as protein ingredients for food and feed applications. The effects of proteolytic enzyme modification of egg-yolk protein preparation (YP) and white protein preparation (WP), obtained as the by-products left during the course of lecithin, lysozyme, and cystatin isolation on their biological and functional properties, were evaluated by treating a commercial Neutrase. The antihypertensive and antioxidative properties of YP and WP hydrolysates were evaluated based on their angiotensin-converting enzyme (ACE)-inhibitory activity and radical scavenging (DPPH) capacity, ferric reducing power, and chelating of iron activity. The functionality of obtained hydrolysates was also determined. Neutrase caused a degree of hydrolysis (DH) of YP and WP by-products: 27.6% and 20.9%, respectively. In each of them, mixture of peptides with different molecular masses were also observed. YP hydrolysate showed high levels of antioxidant activity. The scavenging capacity, ferric reducing power, and chelating capacity were observed at the level: 0.44 μmol/L Trolox mg(-1), 177.35 μg Fe(2+) mg(-1), and 549.87 μg Fe(2+) mg(-1), respectively. YP hydrolysate also exhibited significant ACE-inhibitory activity, in which the level was 59.2 μg. Protein solubility was significantly improved as the DH increased. WP hydrolysate showed high water-holding capacity of 43.2. This study indicated that YP and WP hydrolysates could be used in foods as natural antioxidants and functionality enhancers.
Keywords: Antioxidant activity; bioactive peptides; egg proteins; hydrolysis.
Figures

References
-
- Adebiyi AP, Adebiyi AO, Ogawa T, Muramoto K. Purification and characterization of antioxidative peptides from unfractionated rice bran protein hydrolysates. Int. Food Sci. Technol. 2008;43:35–43.
-
- Ardo Y, Polychroniadou A. Laboratory manual for chemical analysis of cheese. Luxemburg: Publication Office of The European Communities; 1999. Analysis of free fatty acids.
-
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal. Biochem. 1996;293:70–76. - PubMed
-
- Chang CY, Wu KC, Chiang SH. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007;100:1537–1543.
-
- Chen HM, Muramoto K, Yamauchi F. Structural analysis of antioxidative peptides from soybean beta-conglycinin. J. Agric. Food Chem. 1995;43:574–578.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

