Pharmacokinetic and Biodistribution Assessment of a Near Infrared-Labeled PSMA-Specific Small Molecule in Tumor-Bearing Mice
- PMID: 24804103
- PMCID: PMC3997074
- DOI: 10.1155/2014/104248
Pharmacokinetic and Biodistribution Assessment of a Near Infrared-Labeled PSMA-Specific Small Molecule in Tumor-Bearing Mice
Abstract
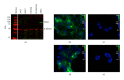
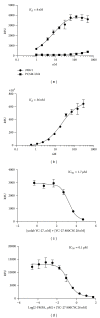
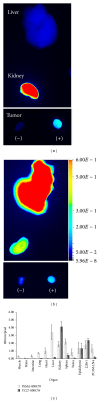
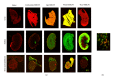
Prostate cancer is the most frequently diagnosed cancer in men and often requires surgery. Use of near infrared (NIR) technologies to perform image-guided surgery may improve accurate delineation of tumor margins. To facilitate preclinical testing of such outcomes, here we developed and characterized a PSMA-targeted small molecule, YC-27. IRDye 800CW was conjugated to YC-27 or an anti-PSMA antibody used for reference. Human 22Rv1, PC3M-LN4, and/or LNCaP prostate tumor cells were exposed to the labeled compounds. In vivo targeting and clearance properties were determined in tumor-bearing mice. Organs and tumors were excised and imaged to assess probe localization. YC-27 exhibited a dose dependent increase in signal upon binding. Binding specificity and internalization were visualized by microscopy. In vitro and in vivo blocking studies confirmed YC-27 specificity. In vivo, YC-27 showed good tumor delineation and tissue contrast at doses as low as 0.25 nmole. YC-27 was cleared via the kidneys but bound the proximal tubules of the renal cortex and epididymis. Since PSMA is also broadly expressed on the neovasculature of most tumors, we expect YC-27 will have clinical utility for image-guided surgery and tumor resections.
Figures

References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer Journal for Clinicians. 2012;62(1):10–29. - PubMed
-
- Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nature Reviews Cancer. 2006;6(9):714–727. - PubMed
-
- Israeli RS, Powell CT, Corr JG, Fair WR, Heston WDW. Expression of the prostate-specific membrane antigen. Cancer Research. 1994;54(7):1807–1811. - PubMed
-
- Silver DA, Pellicer I, Fair WR, Heston WDW, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clinical Cancer Research. 1997;3(1):81–85. - PubMed
-
- Futterer JJ, Barentsz JO. MRI-guided and robotic-assisted prostate biopsy. Current Opinion in Urology. 2012;22:316–319. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

