A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation
- PMID: 24804638
- PMCID: PMC4425293
- DOI: 10.1097/PRS.0000000000000417
A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation
Abstract
Background: Scarring represents a significant biomedical burden in clinical medicine. Mechanomodulation has been linked to scarring through inflammation, but until now a systematic approach to attenuate mechanical force and reduce scarring has not been possible.
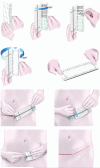
Methods: The authors conducted a 12-month, prospective, open-label, randomized, multicenter clinical trial to evaluate abdominoplasty scar appearance following postoperative treatment with the embrace Advanced Scar Therapy device to reduce mechanical forces on healing surgical incisions. Incisions from 65 healthy adult subjects were randomized to receive embrace treatment on one half of an abdominoplasty incision and control treatment (surgeon's optimal care methods) on the other half. The primary endpoint for this study was the difference between assessments of scar appearance for the treated and control sides using the visual analogue scale scar score.
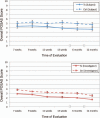
Results: Final 12-month study photographs were obtained from 36 subjects who completed at least 5 weeks of dressing application. The mean visual analogue scale score for embrace-treated scars (2.90) was significantly improved compared with control-treated scars (3.29) at 12 months (difference, 0.39; 95 percent confidence interval, 0.14 to 0.66; p = 0.027). Both subjects and investigators found that embrace-treated scars demonstrated significant improvements in overall appearance at 12 months using the Patient and Observer Scar Assessment Scale evaluation (p = 0.02 and p < 0.001, respectively). No serious adverse events were reported.
Conclusions: These results demonstrate that the embrace device significantly reduces scarring following abdominoplasty surgery. To the authors' knowledge, this represents the first level I evidence for postoperative scar reduction.
Clinical question/level of evidence: Therapeutic, II.
Trial registration: ClinicalTrials.gov NCT01399099.
Figures



References
-
- MedMarket Diligence . Established and Emerging Products, Technologies and Markets in the U.S., Europe, Japan and Rest of the World. MedMarket Diligence; Foothill Ranch, Calif: 2005.
-
- Stuart M. Start-ups, strategics help mend wound repair. Start Up. 2013 May;
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. - PubMed
-
- Cullen KA, Hall MJ, Golosinkiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11:1–25. - PubMed
-
- Centers for Disease Control and Prevention [June 6, 2013];National hospital discharge survey: 2010 table. Procedures by selected patient characteristics—Number by procedure category and age. 2010 Available at: http://www.cdc.gov/nchs/data/ nhds/4procedures/2010pro4_numberprocedurea....
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

